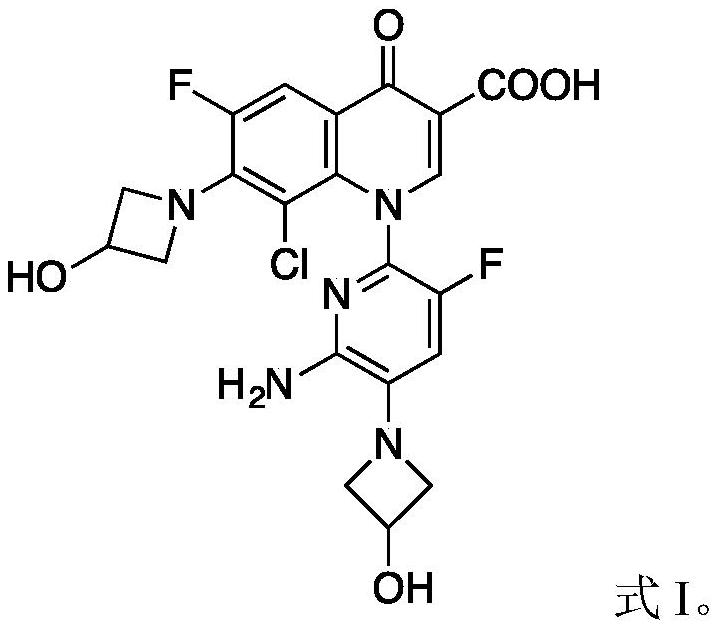
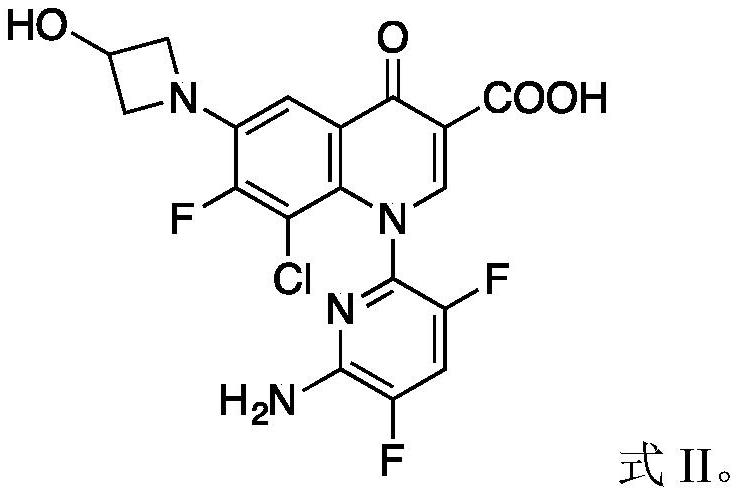
Delafloxacin impurities I and II and product refining method
A technology of delafloxacin and a refining method, which is applied in the field of impurities I and II of delafloxacin and product refining, can solve the problems of difficulty in detecting impurities, and achieve the effects of reducing energy consumption, mild conditions and improving product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 impurity preparation
[0035]
[0036] Add compound 1 (4.050Kg), LiCl (0.8Kg) and N-methylpyrrolidone (15Kg) into a 100L vertical reactor, stir the system well, and slowly add DBU (1.5Kg) dropwise. After the dropwise addition was completed and the reaction was stirred for 2 hours, 3-hydroxyazetidine hydrochloride (1.5Kg) was added, and DBU (4.0Kg) was slowly added dropwise. After the dropwise addition was completed, the internal temperature was controlled at 80°C to continue the reaction for 1 hour. The reaction solution was lowered to 20°C, and 31.6Kg of 10% citric acid aqueous solution was added dropwise at an internal temperature of ≤20°C. During the dropwise addition, a yellow solid was precipitated, centrifuged until dry, and the solid was dried in vacuum to obtain Compound 2 (3.96kg);
[0037] Add compound 2 (3.96Kg) and isopropanol (23.5Kg) into a 100L vertical reactor, and stir to raise the temperature. Add 6% potassium hydroxide aqueous solutio...
Embodiment 2
[0072] Embodiment 2 delafloxacin meglumine salt refining
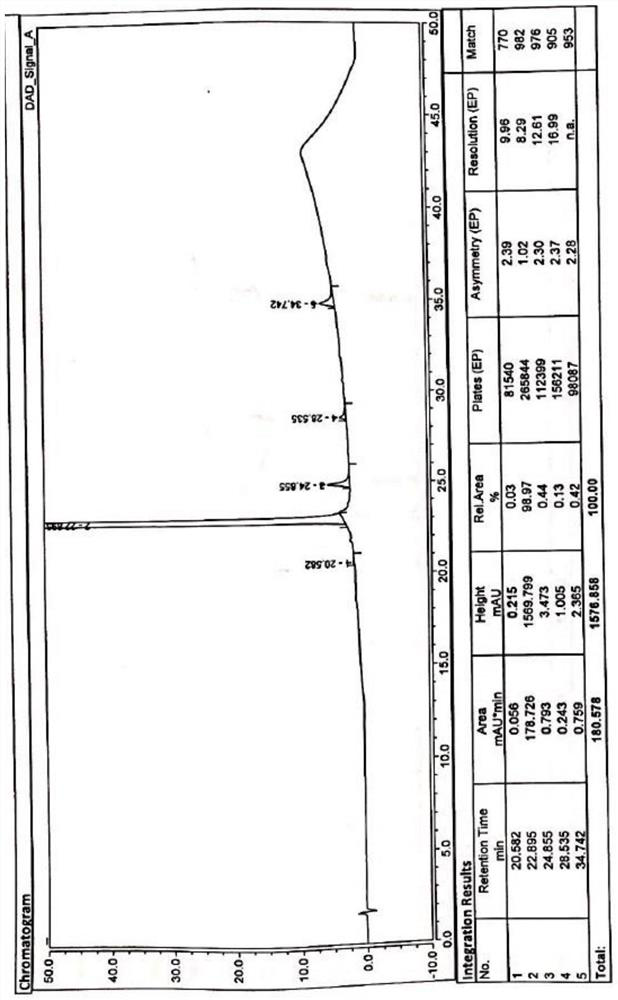
[0073] Weigh delafloxacin meglumine salt (15.0g, 0.023mol) into a 250ml three-necked flask, add 45mL of purified water and 44mL of isopropanol, keep stirring at 60°C for 1h until all solids are dissolved, filter, and cool the filtrate to 5-10°C, slowly add 89mL of isopropanol dropwise, stir and crystallize for 12h, filter with suction, rinse with a small amount of isopropanol, collect the filter cake and dry it in vacuum at 80±5°C for 10h to obtain 12.5g of the refined product with a yield of 83.3 %, detected with the HPLC method described in Example 1, HPLC main component: 99.75%; Impurity I: not detected; Impurity II: 0.14%; Impurity III: not detected; Impurity IV: 0.11%; .
Embodiment 3
[0074] Embodiment 3 delafloxacin meglumine salt refining
[0075] Weigh delafloxacin meglumine salt (15.0g, 0.023mol) into a 250ml three-necked flask, add 45mL of purified water and 44mL of isopropanol, keep stirring at 50°C for 2h, until all solids are dissolved, filter, and cool the filtrate to 5-10°C, slowly add 89mL of isopropanol dropwise, stir and crystallize for 18h, filter with suction, rinse with a small amount of isopropanol, collect the filter cake and dry it in vacuum at 80±5°C for 10h to obtain 11.5g of refined product with a yield of 76.7 %, detected with the HPLC method described in Example 1, impurity I: not detected; impurity II: 0.15%; impurity III: not detected; impurity IV: 0.12%; does not contain other substances.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com