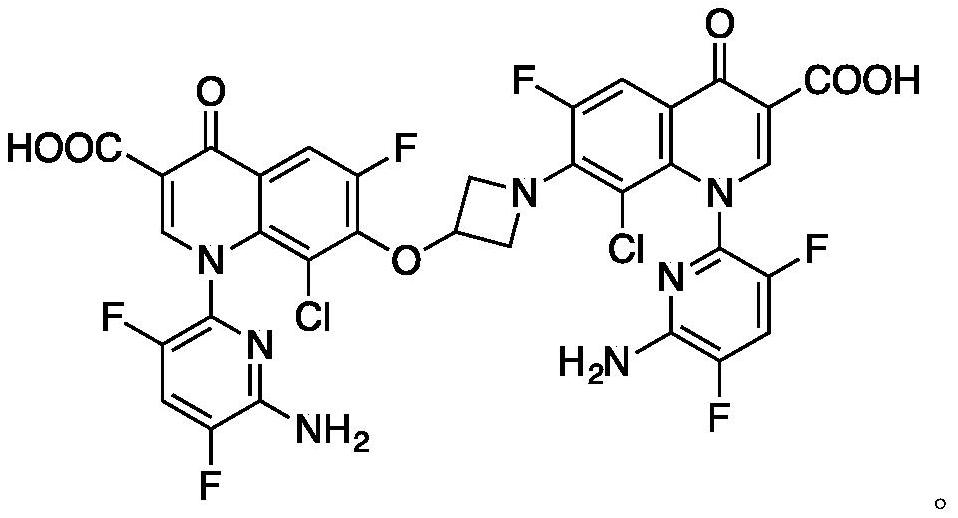
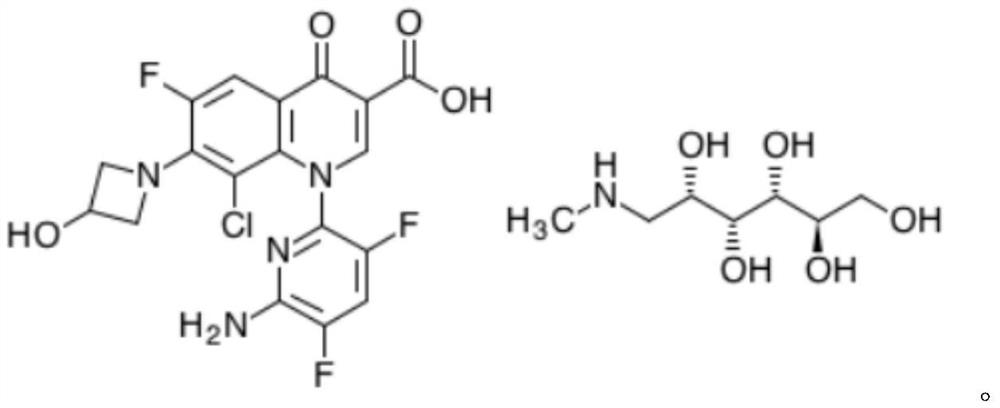
Delafloxacin impurity IV and product refining method
A technology of delafloxacin and its refining method, which is applied in the field of delafloxacin impurity IV and its preparation, can solve problems such as impurity detection is difficult to achieve, and achieve the effects of reducing energy consumption, improving product quality, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 impurity preparation
[0035]
[0036] Add compound 1 (4.050Kg), LiCl (0.8Kg) and N-methylpyrrolidone (15Kg) into a 100L vertical reactor, stir the system well, and slowly add DBU (1.5Kg) dropwise. After the dropwise addition was completed and the reaction was stirred for 2 hours, 3-hydroxyazetidine hydrochloride (1.5Kg) was added, and DBU (4.0Kg) was slowly added dropwise. After the dropwise addition was completed, the internal temperature was controlled at 80°C to continue the reaction for 1 hour. The reaction solution was lowered to 20°C, and 31.6Kg of 10% citric acid aqueous solution was added dropwise at an internal temperature of ≤20°C. During the dropwise addition, a yellow solid was precipitated, centrifuged until dry, and the solid was dried in vacuum to obtain Compound 2 (3.96kg);
[0037] Add compound 2 (3.96Kg) and isopropanol (23.5Kg) into a 100L vertical reactor, and stir to raise the temperature. Add 6% potassium hydroxide aqueous solutio...
Embodiment 2
[0074] Embodiment 2 delafloxacin meglumine salt refining
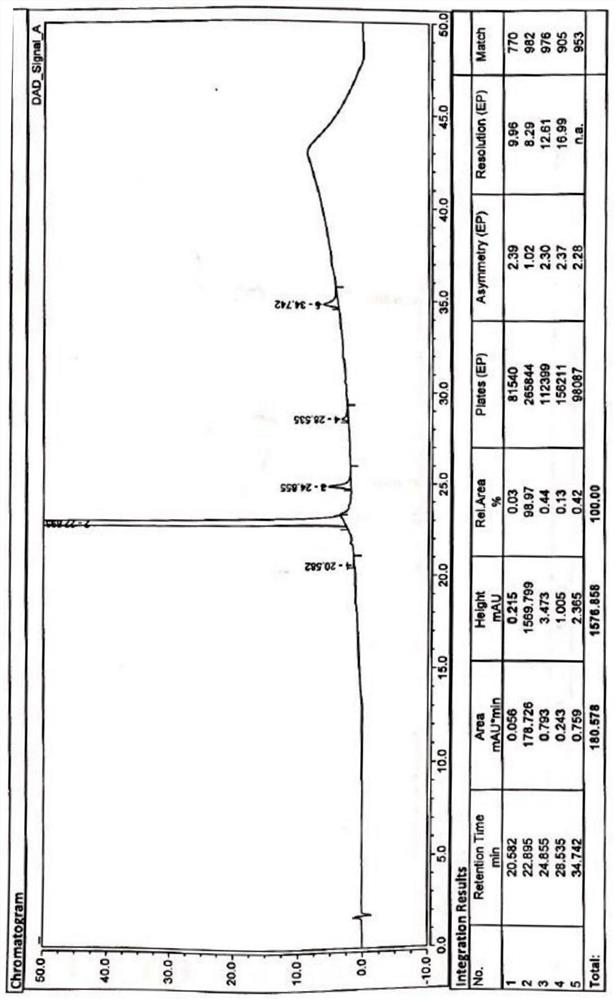
[0075] Weigh delafloxacin meglumine salt (15.0g, 0.023mol) into a 250ml three-necked flask, add 45mL of purified water and 44mL of isopropanol, keep stirring at 60°C for 1h until all solids are dissolved, filter, and cool the filtrate to 5-10°C, slowly add 89mL of isopropanol dropwise, stir and crystallize for 12h, filter with suction, rinse with a small amount of isopropanol, collect the filter cake and dry it in vacuum at 80±5°C for 10h to obtain 12.5g of the refined product with a yield of 83.3 %, detected with the HPLC method described in Example 1, HPLC main component: 99.75%; Impurity I: not detected; Impurity II: 0.14%; Impurity III: not detected; Impurity IV: 0.11%; .
Embodiment 3
[0076] Embodiment 3 delafloxacin meglumine salt refining
[0077] Weigh delafloxacin meglumine salt (15.0g, 0.023mol) into a 250ml three-necked flask, add 45mL of purified water and 44mL of isopropanol, keep stirring at 50°C for 2h, until all solids are dissolved, filter, and cool the filtrate to 5-10°C, slowly add 89mL of isopropanol dropwise, stir and crystallize for 18h, filter with suction, rinse with a small amount of isopropanol, collect the filter cake and dry it in vacuum at 80±5°C for 10h to obtain 11.5g of refined product with a yield of 76.7 %, detected with the HPLC method described in Example 1, impurity I: not detected; impurity II: 0.15%; impurity III: not detected; impurity IV: 0.12%; does not contain other substances.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com