Synthesis method of p-toluenesulfonylurea
A technology of toluenesulfonylurea and p-toluenesulfonamide, which is applied in the field of synthesis of p-toluenesulfonylurea, can solve the problems of non-environmental protection, complicated preparation process, and by-products, and achieve non-toxic and pollution-free operating environment, easy operation The effect of simple steps and low production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
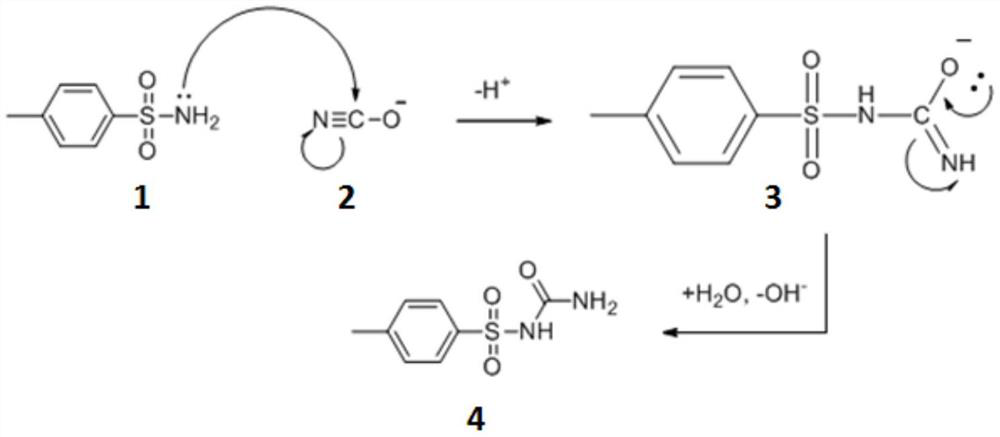
[0025] A synthetic method for p-toluenesulfonylurea, comprising the steps of: adding p-toluenesulfonamide 171g (1mol), ethanol 150mL, sodium cyanate 78g (1.2mol), water 150mL in a there-necked flask, after stirring evenly, heating to Reflux at 78°C, keep warm for 8 hours until the raw materials basically react completely, cool down to below 30°C, filter to remove a small amount of unreacted raw materials that may exist, adjust the pH of the mother liquor to 2 with hydrochloric acid, and filter again to obtain , and dried at 50° C. to obtain 192.6 g of p-toluenesulfonylurea white powder, and the calculated yield was 90%.
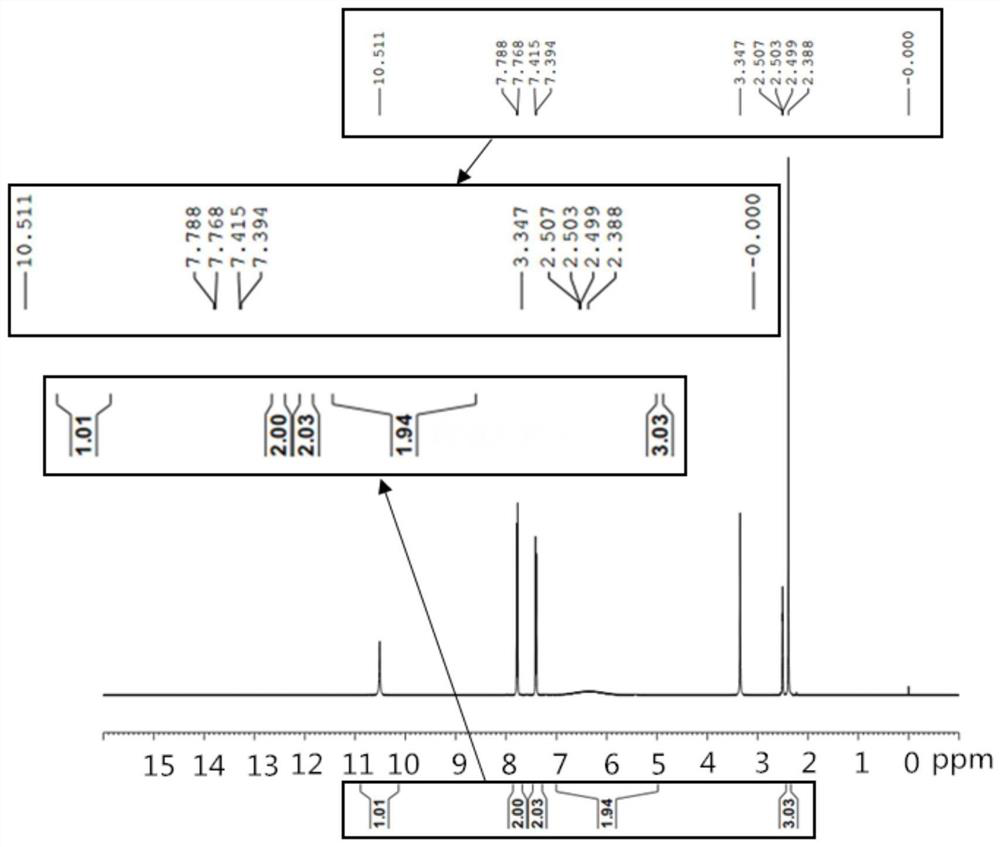
[0026] The p-toluenesulfonylurea prepared in the present embodiment is carried out to the proton nuclear magnetic resonance spectrum test, and the spectrogram is as follows: figure 1 shown by figure 1 It can be seen that the product is p-toluenesulfonylurea.
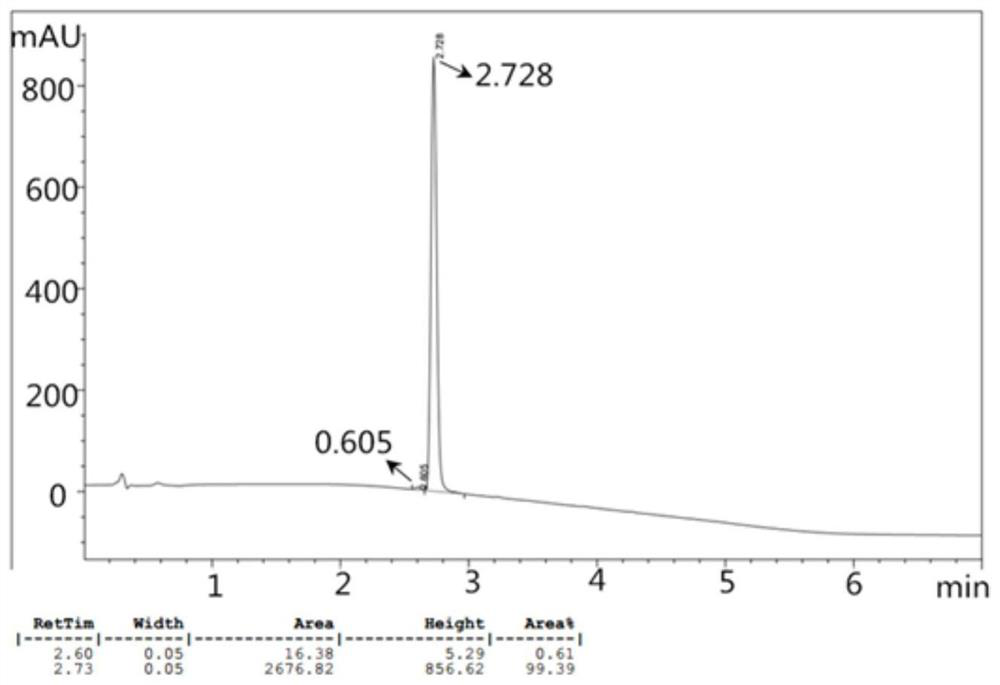
[0027] The p-toluenesulfonylurea prepared in this embodiment is tested by high performance liquid ...
Embodiment 2
[0029] A synthetic method for p-toluenesulfonylurea, comprising the steps of: adding p-toluenesulfonamide 171g (1mol), ethanol 150mL, sodium cyanate 78g (1.2mol), water 150mL in a there-necked flask, after stirring evenly, heating to Reflux at 95°C, keep warm for 5 hours until the raw materials are basically reacted completely, cool down to below 30°C, filter to remove a small amount of unreacted raw materials that may exist, adjust the pH value of the filtered mother liquor to 2 with hydrochloric acid, and filter again to obtain p-toluene The sulfonylurea was dried at 50° C. to obtain 181.9 g of p-toluenesulfonylurea white powder, and the calculated yield was 85%.
[0030] The p-toluenesulfonylurea prepared in this example was subjected to a proton nuclear magnetic resonance spectrum test, and the peak position of the test result product was the same as in Example 1.
[0031] The p-toluenesulfonylurea prepared in this example was tested by high performance liquid chromatograp...
Embodiment 3
[0033] A synthetic method for p-toluenesulfonylurea, comprising the steps of: adding p-toluenesulfonamide 171g (1mol), ethanol 150mL, sodium cyanate 78g (1.2mol), water 150mL in a there-necked flask, after stirring evenly, heating to Reflux at 60°C, keep warm for 7 hours until the raw materials are basically reacted completely, cool down to below 30°C, filter to remove a small amount of unreacted raw materials that may exist, adjust the pH value of the filtered mother liquor to 1 with hydrochloric acid, and filter again to obtain p-toluene The sulfonylurea was dried at 50° C. to obtain 171.2 g of p-toluenesulfonylurea white powder, and the calculated yield was 80%.
[0034] The p-toluenesulfonylurea prepared in this example was subjected to a proton nuclear magnetic resonance spectrum test, and the peak position of the test result product was the same as in Example 1.
[0035] The p-toluenesulfonylurea prepared in this example was tested by high performance liquid chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com