Application of 3-oxo-5 beta-steroid-4-dehydrogenase inhibitor
A dehydrogenase inhibitor, steroid technology, applied in the field of medicine, can solve problems such as no target mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Test of anti-tumor activity
[0026] 1. Experimental samples and experimental methods
[0027] Configuration of the tested sample solution: The test sample is the above compound 1, accurately weigh an appropriate amount of sample, and configure it with DMSO to form a solution of the required concentration for activity testing.
[0028] Cell line and cell subculture: The viability test uses human liver cancer cells HepG2, SMMC7721, Bel7402, prostate cancer PC3 and its paclitaxel-resistant cells, and the cell culture medium is RPMI 1640 +10%FBS+1% (Penicillin-Streptomycin Solution) , Microglioma CHME-5, colon cancer HCT-116 use DMEM medium containing 10% FBS, SW579 cells use 10% FBSMPI-1640 medium, cultured in a 37︒C incubator with 5% carbon dioxide . The cell proliferation inhibitory activity test method adopts the MTT method.
[0029] 2. Experimental results
[0030] Table 1. The inhibitory ability of compound 1 on different tumor cells
[0031]
[0032] 3. Conclusion...
Embodiment 2
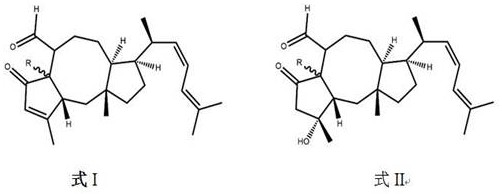
[0034] Example 2 Reverse virtual analysis of compound 1 target protein
[0035] Submit the 4 compounds of formula I to the reverse virtual platform of Beijing Computing Center. The disease target database is composed of the protein structure of human genome druggable genes in the PDB database with complete analysis and partial analysis, and AutoDockVina is used for molecular docking analysis http: / / reversedock.vslead.com / ).
[0036] The compounds are connected to the active site of the target one by one, and the target proteins with the highest scores of the four compounds are all AKR1D1 according to the compound-target interaction energy or pharmacophore matching score.
Embodiment 3
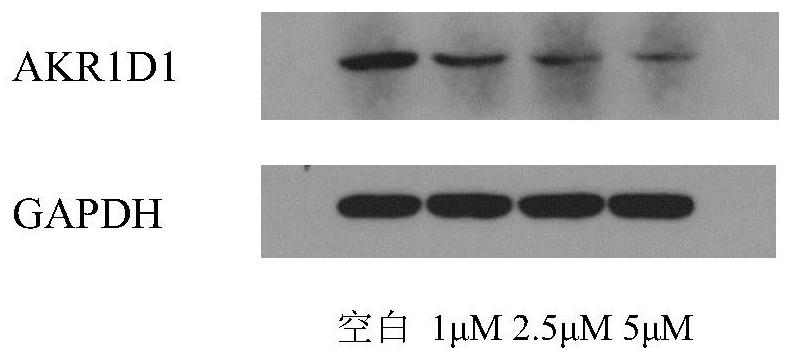
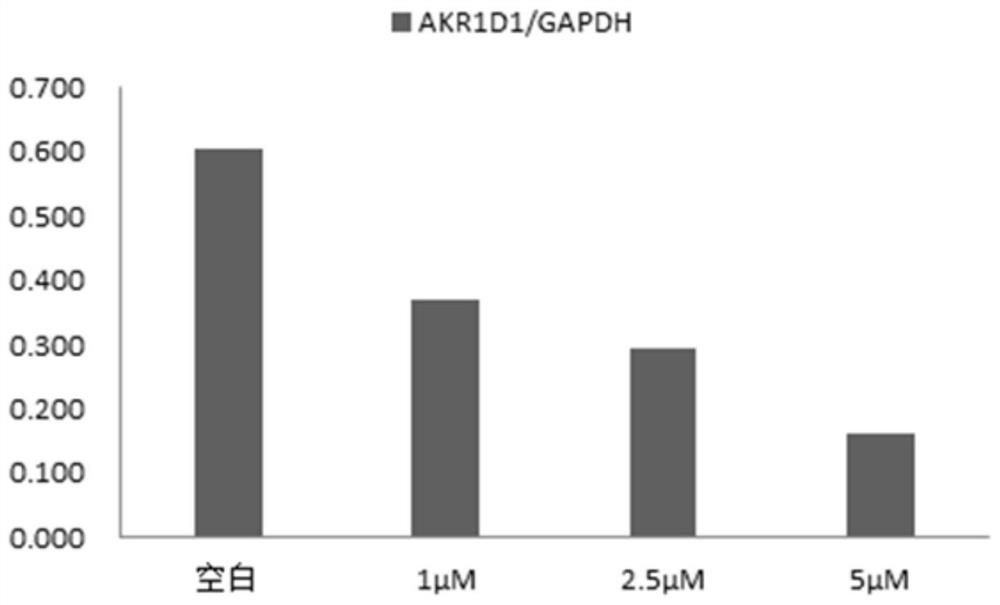
[0037] Example 3 Test to down-regulate AKR1D1 protein level in HepG2 cells
[0038] 1. Experimental samples and experimental methods
[0039] (1) The configuration of the tested sample solution: the test sample is the above compound 1, accurately weigh an appropriate amount of the sample, and use DMSO to configure the solution with the required concentration for the activity test.
[0040] (2) Cell culture
[0041] The HepG2 medium for human liver cancer cells is RPMI 1640 +10%FBS+1% (Penicillin-
[0042] Streptomycin Solution), cultured at 37︒C, 5% carbon dioxide.
[0043] Take HepG2 cells that are in logarithmic growth phase and grow well. They are collected by 0.25% trypsin digestion. The cells are resuspended in RPMI 1640 medium by centrifugation at 12000 rpm for 3 min. The cells are counted on a cell counter, and the cell concentration is diluted with RPMI 1640 medium. 2.5×10 5 cells / ml, connected to a 6-well plate.
[0044] Set up a blank control group without adding any compound,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com