Rapid detection technology of high-sensitivity pathogenic nucleic acid for non-diagnosis and treatment purposes
A detection technology, highly sensitive technology, applied in the direction of recombinant DNA technology, microbe-based methods, microbiological determination/testing, etc., can solve the problems of long detection cycle, limited application, complex kit storage and transportation conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Pathogen nucleic acid sequence determination and primer design
[0024] (1) Take the novel coronavirus as an example
[0025] Get the 2019-nCoV sequence from https: / / www.ncbi.nlm.nih.gov / nuccore / MN908947.3.
[0026] (2) Design of primers for ORF gene and S gene markers of novel coronavirus pneumonia
[0027] ORF forward primer tag: / 5biotin / CG AAG TTG TAG GAG ACA TTA TAC TTAAACC
[0028] ORF reverse primer marker: / 56-FAM / TA GTA AGA CTA GAA TTG TCT ACA TAA GCA GC
[0029] ORF reverse primer marker: 2 / 56-digoxingenine / TA GTA AGA CTA GAA TTG TCT ACA TAAGCA GC
[0030] S forward primer tag: / 5biotin / AG GTT TCA AAC TTT ACT TGC TTT ACA TAG A
[0031] S reverse primer marker: / 56-FAM / TC CTA GGT TGA AGA TAA CCC ACA TAA TAAG
[0032] S reverse primer marker: 2 / 56-digoxingenine / TC CTA GGT TGA AGA TAA CCC ACA TAATAAG
Embodiment 2
[0033] Example 2 Pathogen S gene and ORF gene sequence amplification template
[0034] (1) RNA template;
[0035] (2) The repolymerase amplification kit was purchased from TwistDx, https: / / www.twistdx.co.uk / en / products / product / twistamp-nfo;
[0036] (3) Reverse transcriptase: protoSciptII reverse transcriptase, M0368L, New England biotinlabs, https: / / www.neb.com / products / m0368-protoscript-ii-reverse-transcriptase#product%20information;
[0037] (4) The repolymerase amplification (RPA) reaction system is as shown in Table 1;
[0038] (5) After the system is assembled, put it in a water bath at 42°C for 20 minutes.
[0039] Table 1
[0040]
[0041]
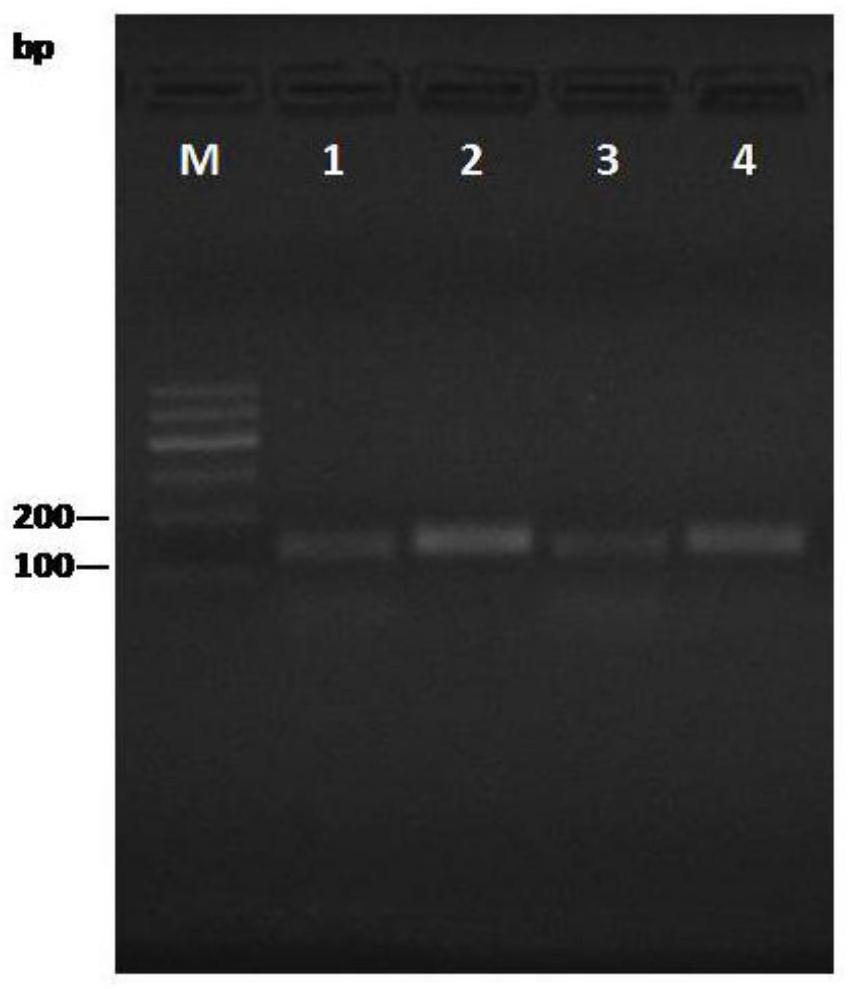
[0042] Amplification was carried out separately in six groups, four of which were used as experimental groups, and the systems used for amplification were numbered system 1, system 2, system 3 and system 4, respectively, and the two groups were used as blank controls, respectively numbered control group 1 and control group...
Embodiment 3
[0043] The PCR of embodiment 3 recombinase amplification products
[0044] The amplified products of systems 1 to 4 in Example 2 were re-amplified by PCR and then developed with colloidal gold.
[0045] (1) PCR enzyme ExTaq (Takara);
[0046] (2) The PCR reaction system is as shown in Table 2;
[0047] (3) After the system is assembled, 95°C for 3min; 95°C for 30s, 55°C for 30s, 72°C for 10s, 28cycles; 72°C for 5min.
[0048] Table 2
[0049]
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com