Organic electroluminescent material taking arylamine structure as central skeleton as well as preparation method and application of organic electroluminescent material
A technology of electroluminescent material and central skeleton, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as low luminous efficiency and inability to meet high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
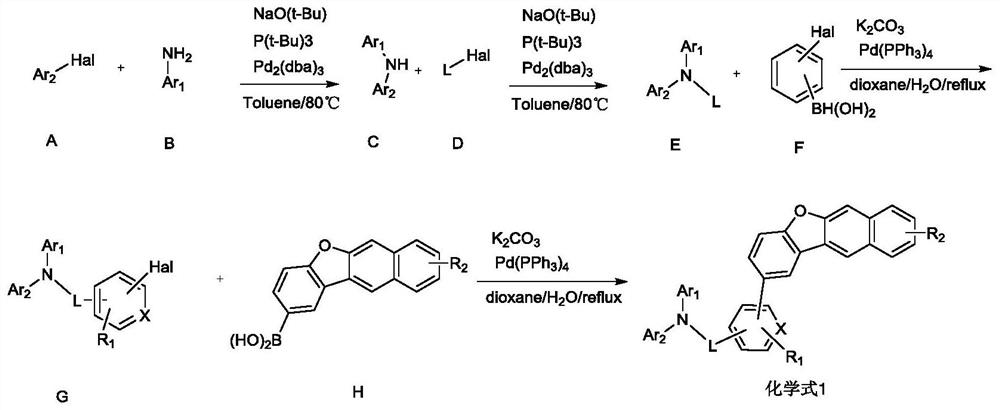
[0052] The present invention provides a method for preparing an organic electroluminescent material described in the above technical solution, comprising the following steps:
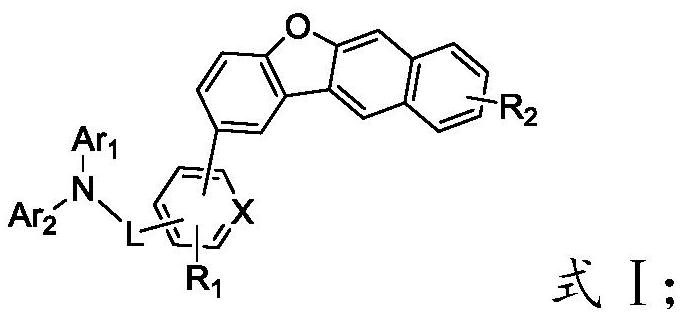
[0053] reacting a substance having a structure of formula G with a substance having a structure of formula H to obtain an organic electroluminescent material having a structure of formula I;
[0054] The Hal is selected from halogen; preferably selected from Br or Cl;
[0055]
[0056] In the present invention, Ar in formula G 1 and Ar 2 , R 1 The selection of the substituents of L and X is consistent with the above-mentioned technical scheme, and will not be repeated here.
[0057] The substance having the structure of formula G is preferably prepared according to the following method:
[0058] Under the protection of nitrogen, the substance with formula E and the substance with formula F are reacted in the presence of potassium carbonate and tetrakistriphenylphosphine palladium to obtain the ...
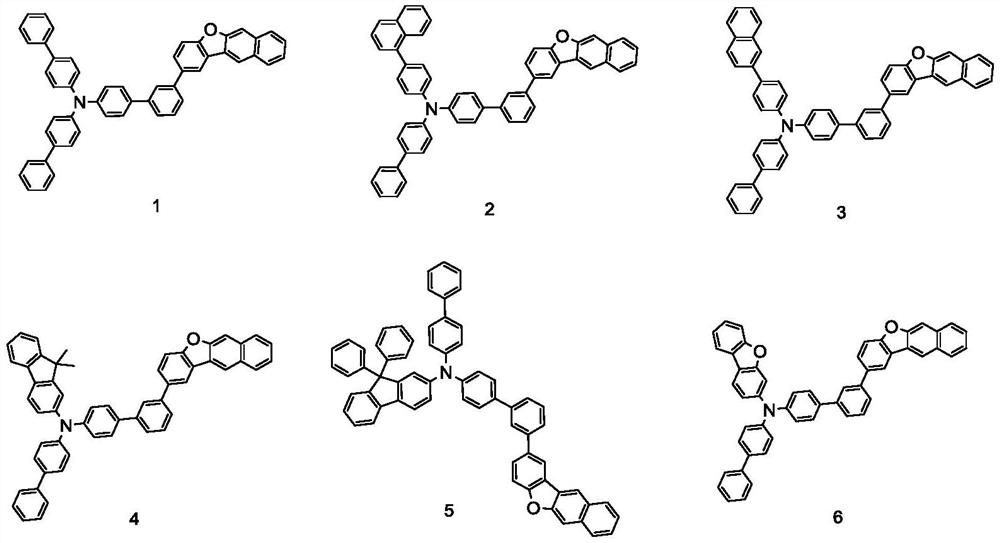
Embodiment 1
[0088]
[0089] in N 2 Under protection, raw material A-1 (11.65g, 50mmol) and raw material B-1 (8.46g, 50mmol) were added to 120ml of toluene solution and stirred. After stirring at 50°C for 15min, sodium tert-butoxide (9.61g, 100mmol) was added ), tri-tert-butylphosphine (0.20g, 1mmol), tris(dibenzylideneacetone) dipalladium (0.46g, 0.5mmol), after the addition was completed, it was heated to 80°C for reaction, and the reaction time was 5 hours. After the reaction was completed, , the solution was cooled to room temperature, and after suction filtration, the concentrated product was recrystallized from 100ml toluene solution to obtain intermediate C-1 (13.2g, yield: 82.14%).
[0090]
[0091] in N 2 Under protection, intermediate C-1 (13.0g, 40.45mmol) and raw material D-1 (7.74g, 40.45mmol) were added to 130ml of toluene solution and stirred at 50°C for 15min, then sodium tert-butoxide (9.61 g, 80.9mmol), tri-tert-butylphosphine (0.16g, 0.81mmol), tris(dibenzylidene...
Embodiment 2
[0097]
[0098] in N 2 Under protection, A-5 (19.86g, 50mmol) and B-5 (8.46g, 50mmol) were added to 200ml of toluene solution and stirred. After stirring at 50°C for 15min, sodium tert-butoxide (9.61g, 100mmol) was added, Tri-tert-butylphosphine (0.20g, 1.0mmol) and tris(dibenzylideneacetone)dipalladium (0.46g, 0.5mmol) were added, heated to 80°C, after the reaction was completed, the solution was cooled to room temperature, and suction filtered The concentrated product was recrystallized from 200ml toluene solution to obtain intermediate C-5 (19.52g, yield: 80.39%).
[0099]
[0100] in N 2 Under protection, raw material C-5 (19.5g, 40.15mmol) and raw material D-5 (7.69g, 40.15mmol) were added to 200ml of toluene solution and stirred at 50°C for 15min, then sodium tert-butoxide (7.72g , 80.3mmol), tri-tert-butylphosphine (0.18g, 0.9mmol), tris(dibenzylideneacetone) dipalladium (0.37g, 0.40mmol) were added, heated to 80°C, after the reaction was completed, after suctio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com