Application and preparation method of gamma-carbolin-hydroxamic acid anti-tumor metastasis compound
An anti-tumor metastasis, carboline hydroxime technology, applied in the field of biomedicine, can solve problems such as poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis and characterization of γ-carboline hydroxamic acid compounds.
[0031] In this example, 1 H-NMR was measured with Bruker AVANCE Ⅲ HD 600 mega-NMR spectrometer; MS was measured with Agilent 6440 Triple Quad LC / MS instrument, and all were ESI methods unless otherwise noted; all solvents were re-distilled before use, and the used All the anhydrous solvents were obtained by drying according to the standard method; except for the instructions, all the reactions were carried out under the protection of argon and followed by TLC, and the post-treatment was washed with saturated saline and dried with anhydrous magnesium sulfate; the product Purification uses silica gel (200-300 mesh) column chromatography unless otherwise specified; the silica gel used, including 200-300 mesh and GF254, are produced by Qingdao Hailang Silica Gel Desiccant Co., Ltd.
[0032] Concrete synthetic steps are as follows:
[0033] (1) Dissolve phenylhydrazine hydrochloride (1000 ...
Embodiment 2
[0071] Example 2 Inhibition of HDAC1 Enzyme Activity by Compounds QY01-QY08
[0072] Using Ac-Lys-Tyr-Lys(Ac)-AMC as substrate, use fluorescence detection method to detect enzyme activity in 96-well or 384-well flat-bottom microplate: Substrate Ac-Lys-Tyr-Lys(Ac)- After AMC was deacetylated by HDAC1, the product AMC obtained by hydrolysis with trypsin emitted 460 nm fluorescence under excitation of 355 nm by a fluorescence detector. After adding the inhibitor, it will affect the intensity of the fluorescence. By detecting the change of the fluorescence signal with time, the initial velocity of the reaction is calculated, and the IC is calculated. 50 , with SAHA as the positive control, the results are shown in Table 1:
[0073] Table 1 Inhibition of HDAC1 by compounds QY01-QY08
[0074]
[0075] It can be seen from Table 1 that compounds QY03-QY08 have certain inhibitory activity on HDAC1, which is significantly better than SAHA, especially the inhibitory activity of QY08...
Embodiment 3
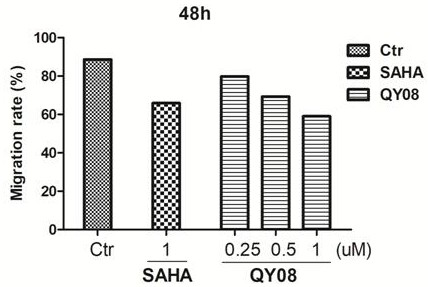
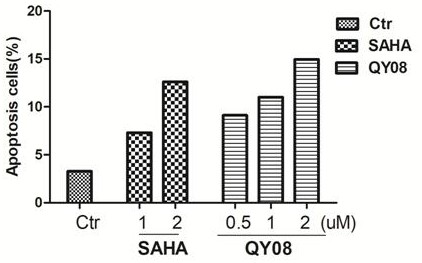
[0076] Example 3 Effects of different compounds on tumor cell proliferation.
[0077] Select compounds QY06-QY08 with better inhibitory activity on HDAC1, use MDA-MB-231, H157, A549, and MCF-7 as target cells, and use Beas-2B and L-02 cells as non-target cells (normal cells), The cell viability was detected by the SRB method: different cells were divided into 6×10 3 Each well was inoculated into a 96-well plate, and after conventional culture for 24 hours, the compound in Example 1 was added in sequence at different concentrations, and three replicate wells were set up for each group. After continuing to culture for 48 h, the cells were fixed, stained with SRB, and the 96-well plate was placed in a microplate reader, and the OD value was measured at a wavelength of 440 nm. Statistical analysis of the effect of drugs on the survival rate of different cells, the results are shown in Table 2:
[0078] Table 2 IC of different compounds on different cell proliferation 50 value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com