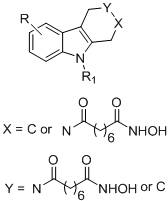
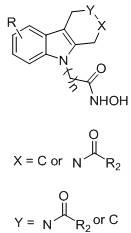
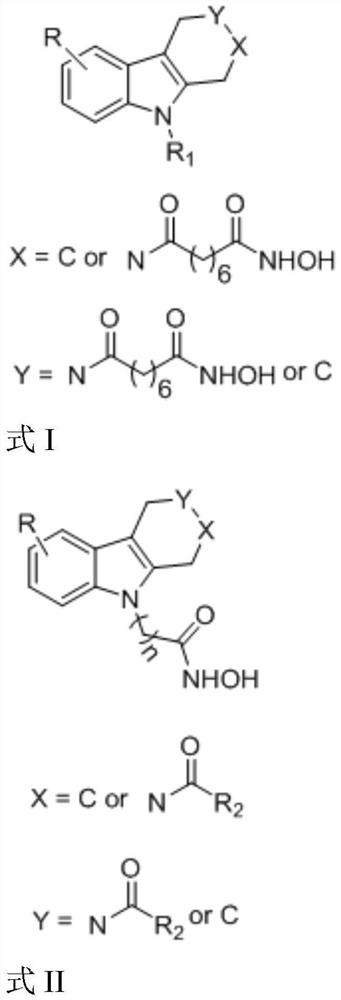
Hydroxamic acid histone deacetylase inhibitor containing tetrahydrocarboline structure as well as preparation method and application of hydroxamic acid histone deacetylase inhibitor
A technology of tetrahydrocarboline and hydroxime, applied in the field of biomedicine, can solve problems such as affecting enzyme activity, changing DNA replication fidelity, etc., and achieve the effect of good inhibition activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0028] Example 1-1, 8-(9-ethyl-1,3,4,9-tetrahydro-2h-pyridine[3,4-b]indol-2-yl)-N-hydroxy-8-oxo Preparation of octanamide (YH01)
[0029] Take tryptamine hydrochloride and paraformaldehyde, stir in water, add 3M sodium acetate aqueous solution and reflux for more than 4 hours, add sodium hydroxide aqueous solution to adjust to alkaline, extract, and evaporate to dryness to obtain compound 2 as a yellow solid.
[0030] Tetrahydro-β-carboline (compound 2) was dissolved in 1,4-dioxane, suberic anhydride was added, and the mixture was heated to reflux for about 5 hours, and intermediate 3 was isolated. Intermediate 3 is dissolved in methanol, 3-4 drops of thionyl chloride are added dropwise, and the reaction system is heated to reflux. After 5 hours, the excess solvent was removed under reduced pressure to obtain the crude product 4, which was passed through a silica gel column after conventional post-treatment and used for the next reaction.
[0031] The obtained compound 4 was...
Embodiment 1-2
[0035] Example 1-2, 8-(8-bromo-5-ethyl-1,3,4,5-tetrahydro-2hydro-pyridin[4,3-b]indol-2-yl)-N- Preparation of Hydroxy-8-oxooctamide (YH10)
[0036] The bromophenylhydrazine hydrochloride and 4-piperidone hydrochloride monohydrate were stirred in ethanol, the reaction was refluxed, sodium hydroxide aqueous solution was added to make it alkaline, and the yellow solid was obtained by suction filtration, that is, compound 7.
[0037] Tetrahydro-γ-carboline (compound 7) was dissolved in 1,4-dioxane, suberic anhydride was added, and the mixture was heated to reflux for about 5 hours, and intermediate 8 was isolated. Intermediate 8 was dissolved in methanol, 3-4 drops of thionyl chloride were added dropwise, and the reaction system was heated to reflux. After 5 hours, the excess solvent was removed under reduced pressure to obtain crude product 9, which was subjected to conventional post-treatment and passed through a silica gel column for the next reaction.
[0038] The obtained co...
Embodiment 1-3
[0042] Example 1-3, 5-(2-benzoyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indol-9-yl)-N-hydroxypentanamide Preparation of (YH19)
[0043]Dissolve HATU and acetic acid in DMF, stir in ice bath for half an hour, add compound 2 (4H-β-carboline) to react overnight at room temperature, extract with EtOAc after the reaction, and pass through silica gel column after routine post-treatment to obtain compound 11.
[0044] Compound 11 was dissolved in DMF, and NaH was added under ice bath to remove hydrogen for half an hour, then methyl 4-bromobutyrate was added to react at room temperature for three hours, extracted with EtOAc after the reaction, and passed through a silica gel column after conventional post-treatment to obtain compound 12.
[0045] KOH was added to the methanol solution of hydroxylamine hydrochloride at 40°C and kept for 10min, then the reaction system was cooled to 0°C and filtered, the ester (compound 12) was added to the filtrate, and then KOH was added, and the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com