Preparation method of trimethylsilanol
A technology of trimethylsilanol and hexamethyldisilazane, which is applied in the field of preparation of trimethylsilanol, can solve problems such as complex use of raw materials, insufficient environmental protection, and complicated processes, and achieve single raw materials and easy expansion The effect of less production and consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
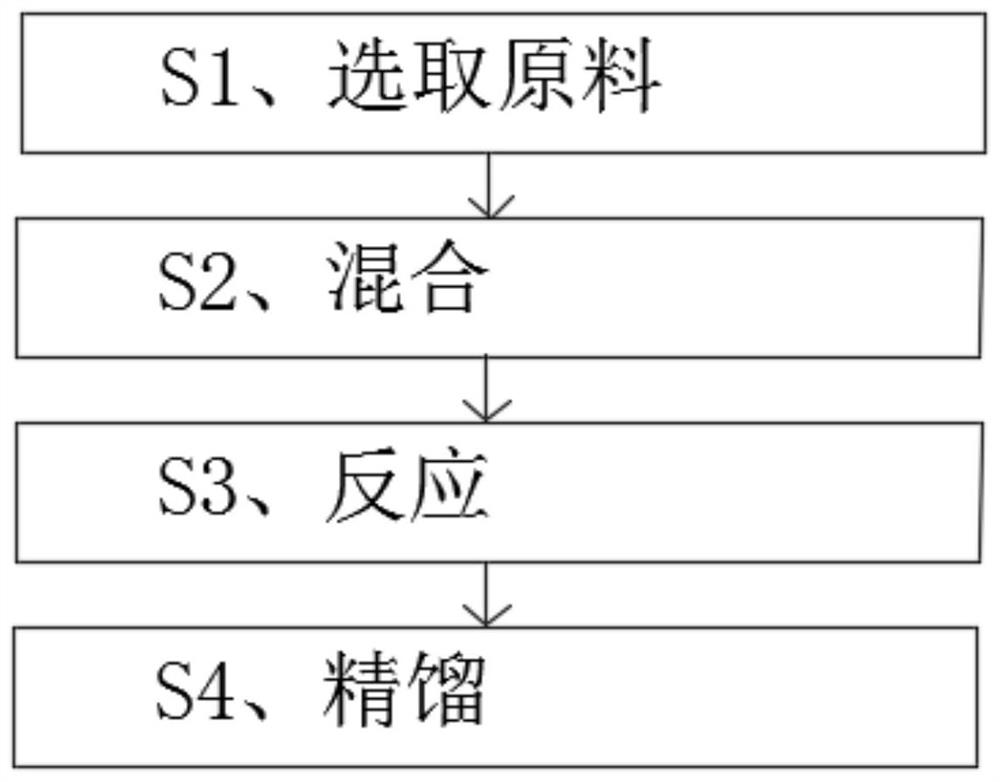
[0028] refer to figure 1 , a preparation method of trimethylsilanol, specifically comprising the following steps:
[0029] S1. Select raw materials: select the following components by weight for use: 450 parts of hexamethyldisilazane, 45 parts of glacial acetic acid, and 90 parts of water;
[0030] S2. Mixing: Add water and glacial acetic acid into a 1000ml four-necked bottle, stir it evenly after mixing, and then heat the mixture to 60°C;
[0031] S3, reaction: add hexamethyldisilazane dropwise to the mixed solution obtained in S2, and react for 2 hours;
[0032] S4. Rectification: distill the solution obtained in S3 to obtain trimethylsilanol with a purity of more than 98%.
[0033] Among them, the ammonia gas produced during the S3 reaction is absorbed and treated by the tail gas recovery device.
[0034] Wherein, when S3 reacts, use HNMR detection means to confirm that the reaction generates trimethylsilanol.
[0035] Wherein, during S4 distillation, the distillation t...
Embodiment 2
[0039] refer to figure 1 , a preparation method of trimethylsilanol, specifically comprising the following steps:
[0040] S1. Select raw materials: select the following components by weight for use: 480 parts of hexamethyldisilazane, 45 parts of glacial acetic acid, and 100 parts of water;
[0041] S2. Mixing: Add water and glacial acetic acid into a 1000ml four-necked bottle, stir it evenly after mixing, and then heat the mixture to 65°C;
[0042] S3. Reaction: add hexamethyldisilazane dropwise to the mixed solution obtained in S2, and react for 2.4 hours;
[0043] S4. Rectification: distill the solution obtained in S3 to obtain trimethylsilanol with a purity of more than 98%.
[0044] Among them, the ammonia gas produced during the S3 reaction is absorbed and treated by the tail gas recovery device.
[0045] Wherein, when S3 reacts, use HNMR detection means to confirm that the reaction generates trimethylsilanol.
[0046] Wherein, during S4 distillation, the distillatio...
Embodiment 3
[0049] refer to figure 1 , a preparation method of trimethylsilanol, specifically comprising the following steps:
[0050] S1. Select raw materials: select the following components by weight for use: 520 parts of hexamethyldisilazane, 45 parts of glacial acetic acid, and 100 parts of water;
[0051] S2. Mixing: Add water and glacial acetic acid into a 1000ml four-necked bottle, stir it evenly after mixing, and then heat the mixture to 68°C;
[0052] S3. Reaction: add hexamethyldisilazane dropwise to the mixed solution obtained in S2, and react for 3 hours;
[0053] S4. Rectification: distill the solution obtained in S3 to obtain trimethylsilanol with a purity of more than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com