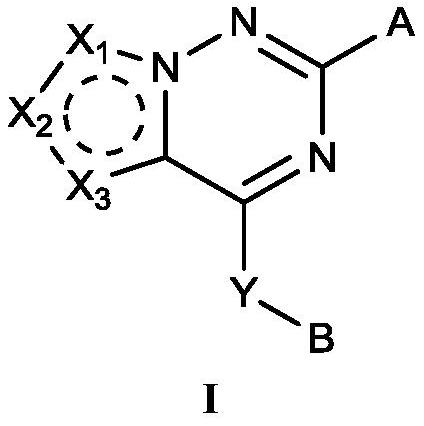
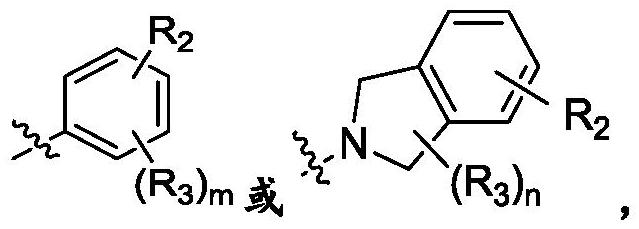
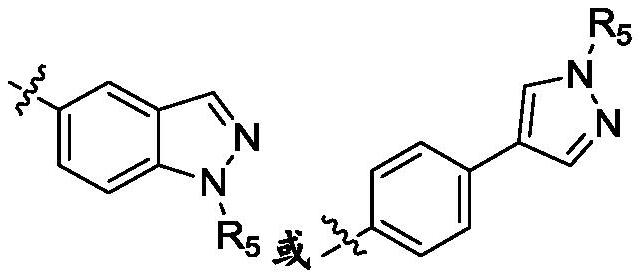
ROCK inhibitor as well as preparation method and application thereof
A CH2, solvate technology, applied in the field of compounds that can inhibit ROCK activity and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Embodiment 1, the preparation of compound (QR001)
[0186]
[0187] 1.1 Preparation of compound (QR001-SM1)
[0188] Potassium carbonate (18g, 126mmol) was added to a solution of isopropylamine (5g, 84mmol) in dichloromethane (20mL) under ice-cooling, and then chloroacetyl chloride (12g, 100.8mmol) was slowly added dropwise, and reacted overnight at room temperature. Water was added, extracted with ethyl acetate, and the organic phase was dried and concentrated under reduced pressure to obtain 9 g of white solid.
[0189] 1 H NMR (DMSO-d 6 , 400MHz): δ8.04(s, 1H), 3.98(s, 2H), 3.85-3.80(m, 1H), 1.07(d, J=6.4Hz, 6H).
[0190] 1.2 Preparation of compound (QR001-3)
[0191] To a solution of compound QR001-SM1 (3 g, 13.6 mmol) in DMF (30 mL) was added potassium carbonate (2.8 g, 20.4 mmol) and 3-hydroxyphenylboronic acid pinacol ester (2.8 g, 20.4 mmol, CAS: 214360 -76-6), the addition was completed, and reacted at 80°C for 16 hours. Water (50 mL) was added, extrac...
Embodiment 2
[0200] Embodiment 2, the preparation of compound (QR003) and (QR004)
[0201]
[0202] 2.1 Preparation of Compounds (QR003-2) and (QR003-2A)
[0203] 2,4-Dichloropyrrole[2,1-f][1,2,4]triazine (1.2g, 6.4mmol) was dissolved in acetonitrile (40mL), and 1-chloromethyl- 4-Fluoro-1,4-diazobicyclo2.2.2-octanebis(tetrafluoroborate) salt (Selectfluor) (CAS: 140681-55-6) (2.5g, 7.06mmol) Fluorinated reagent, addition complete After stirring at room temperature for 2 hours, it was heated to 80° C., and the reaction was continued for 16 hours. After cooling and concentrating, it was purified by silica gel column chromatography to obtain 980 mg of the fluorinated product as a yellow solid, which was identified as a mixture of QR003-2 and QR003-2A by NMR.
[0204] 2.2 Preparation of compound (QR003-SM2)
[0205] 5-Nitroindazole (1.63 g, 10 mmol, CAS: 5401-94-5) was dissolved in 100 mL of anhydrous dichloromethane, and TEA (5 mL) and 2-(trimethylsilyl)ethane were added at 0 °C Oxymeth...
Embodiment 3
[0220] Embodiment 3, the preparation of compound (QR005)
[0221]
[0222] 3.1 Preparation of compound (QR005-2)
[0223] Compound QR001-2 (500mg, 1.299mmol), 3-hydroxyphenylboronic acid (213mg, 1.55mmol, CAS: 87199-18-6), K 3 PO 4 (550mg, 2.59mmol), Pd(OAc) 2 (30mg, 0.132mmol) and XPhos (61mg, 0.128mmol) were added to dioxane (20mL) and water (3mL), stirred overnight at 100°C. After the reaction, the reaction solution was concentrated under reduced pressure and purified by column chromatography to obtain 200 mg of a yellow solid.
[0224] LC-MS: [M+1] + = 343.1.
[0225] 3.2 Preparation of compound (QR005-3)
[0226] Compound QR005-2 (180mg, 0.52mmol) and dimethylaminopyridine (6mg, 0.05mmol) were dissolved in dichloromethane (20mL), and Boc anhydride (170mg, 0.78mmol) was added under ice cooling. After stirring at room temperature for 4 hours, the reaction was completed. Water and DCM were added for extraction, the organic phase was washed with saturated brine, dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com