Sustained and controlled release preparation and preparation method and preparation of in situ tumor immune combination therapy drug application
A technology of controlled release preparation and immunogenicity, which is applied in the field of biomedical nanomaterials, can solve the problems of increasing drug toxicity and side effects, poor drug targeting, and toxic and side effects, and achieves reduction of toxic side effects, high compliance, and high drug loading capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh the prescription according to Table 1, put soybean lecithin (SPC100), sorbitan monooleate (SMO), tocopheryl acetate (TA) and absolute ethanol with different mass ratios in 10mL EP tubes (wherein control TA 10 parts by mass), sealed with sealing glue, then placed on a multi-point magnetic stirrer to stir overnight at 1000 rpm / min, and then placed in an ultrasonic instrument to remove bubbles by ultrasonication to obtain a clear and transparent light yellow liquid crystal precursor solution. Remove the needle part of the 1ml syringe, absorb 0.1ml of the liquid crystal precursor, reinstall the needle of the syringe, inject the liquid crystal precursor into the PBS buffer, check whether it can be injected successfully and observe whether it can form a semi-solid gel and record it. gel time.
[0039] It can be found from Table 1 that with the increase of absolute ethanol, it is easier to form a clear and transparent liquid crystal precursor, and the fluidity is better,...
Embodiment 2
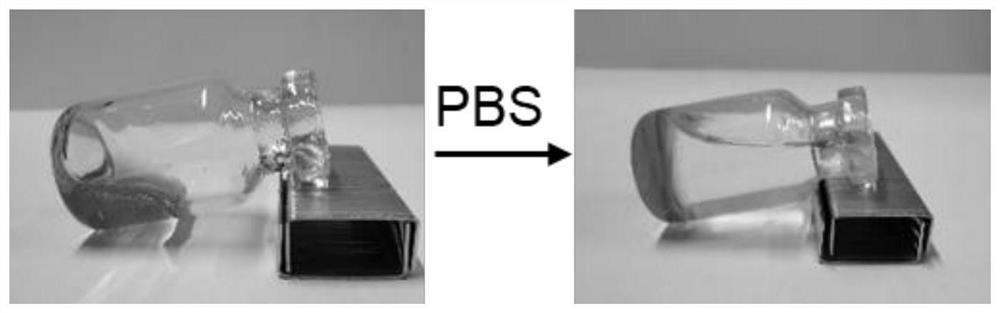
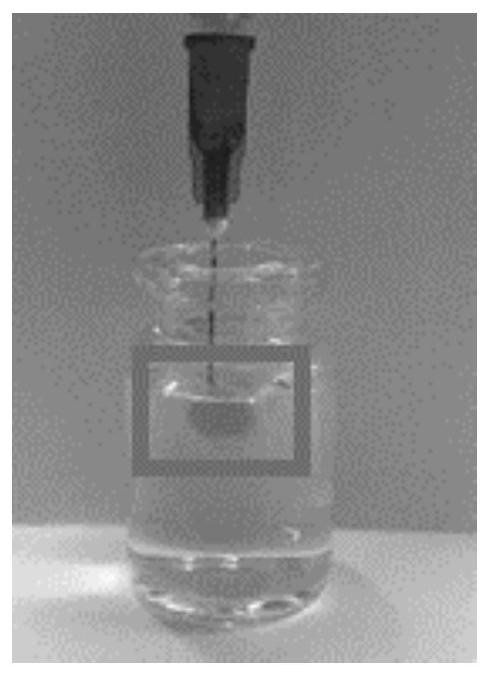
[0043] On the basis of Example 1, we weighed 45 parts by mass of SPC100, 35 parts by mass of SMO, 10 parts by mass of TA and 10 parts by mass of Ethanol to prepare a blank liquid crystal precursor. After the clear and transparent liquid crystal precursor solution was formed, take Part of it was added to a blank ampoule, and the fluidity of the liquid crystal precursor was observed by tilting the ampoule. Then add a certain amount of PBS buffer solution, and tilt the ampoule again after 10 minutes to observe the fluidity of the liquid crystal precursor at the bottom, the results are as follows figure 1 As shown, a semi-solid gel was formed after 10 min. In addition, remove the needle part of the 1ml syringe, absorb 0.1ml of the liquid crystal precursor, reinstall the needle of the syringe, and inject the liquid crystal precursor into the PBS buffer, the results are as follows figure 2 The gel precursors shown form gels immediately upon exposure to aqueous media.
Embodiment 3
[0045] Separately weigh 45 parts by mass of SPC100, 45 parts by mass of GDO, and 10 parts by mass of ethanol in a 10mL EP tube, seal it with sealing glue, and then place it on a multi-point magnetic stirrer to stir overnight at 1000rpm / min, and then place it in an ultrasonic instrument to sonicate Remove the bubbles to clear and transparent light yellow liquid crystal precursor solution. Remove the needle part of the 1ml syringe, absorb 0.1ml of the liquid crystal precursor, reinstall the needle of the syringe and push the liquid crystal precursor into the PBS buffer, the gel precursor will form a gel immediately when it meets water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com