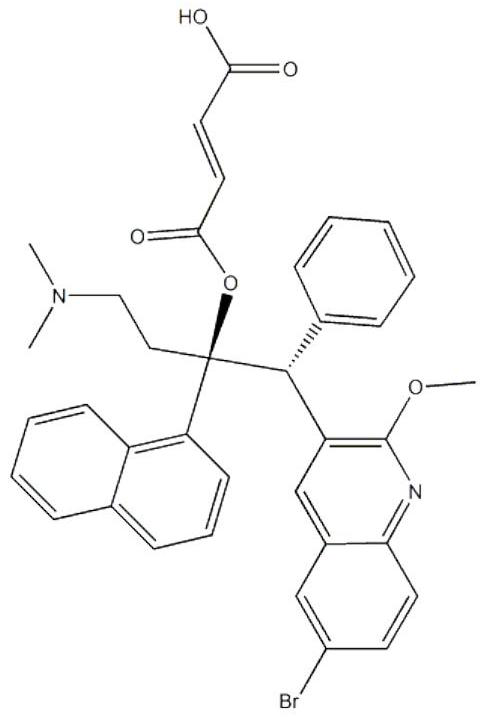
Bedaquiline medicinal preparation
A technology of bedaquiline pharmacy and bedaquiline, which is applied in the direction of antibacterial drugs, pill delivery, pharmaceutical formulations, etc., can solve the problems of poor dissolution effect of bedaquiline, and achieve increased bioavailability and high dissolution rate , good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
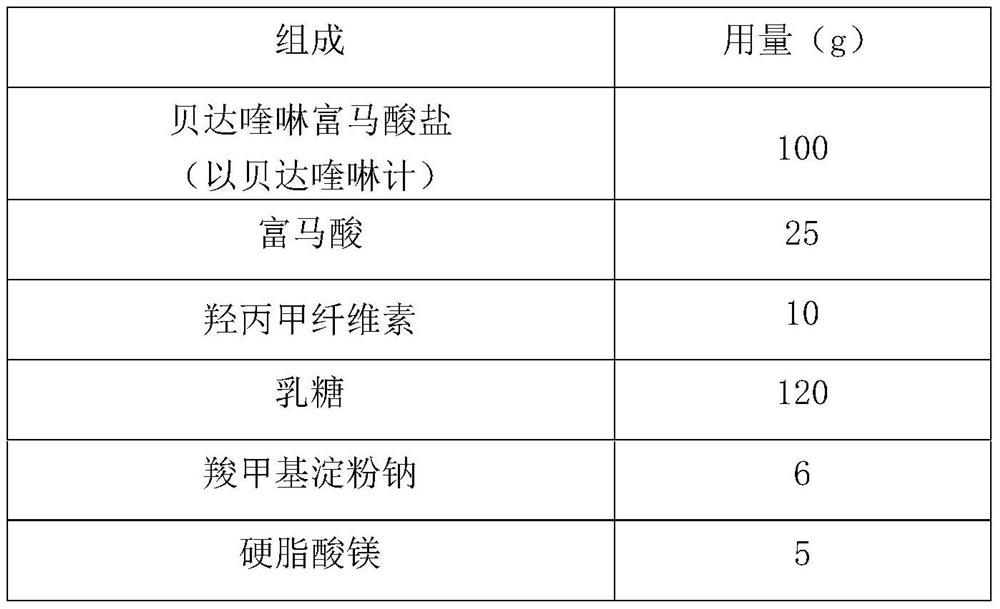
Embodiment 1
[0022]
[0023] Preparation:
[0024] (1) Preparation of bedaquiline solid composition:
[0025] (i) fumaric acid and hypromellose were added to 220mL of ethanol aqueous solution containing 80% ethanol and dissolved to make a mixed solution;
[0026] (ii) In the bedaquiline fumarate of 38 μm in the particle diameter d0.9 placed in the fluidized bed, spray the above-mentioned mixed solution, granulate, dry, and granulate to obtain the bedaquiline solid composition , wherein, the weight ratio of particles with a particle size smaller than 150 μm in the solid composition is 60% (w / w).
[0027] (2) Preparation of bedaquiline preparation:
[0028] The bedaquiline pharmaceutical preparation is obtained by mixing the above-mentioned bedaquiline solid composition with lactose, sodium carboxymethyl starch and magnesium stearate and pressing into tablets.
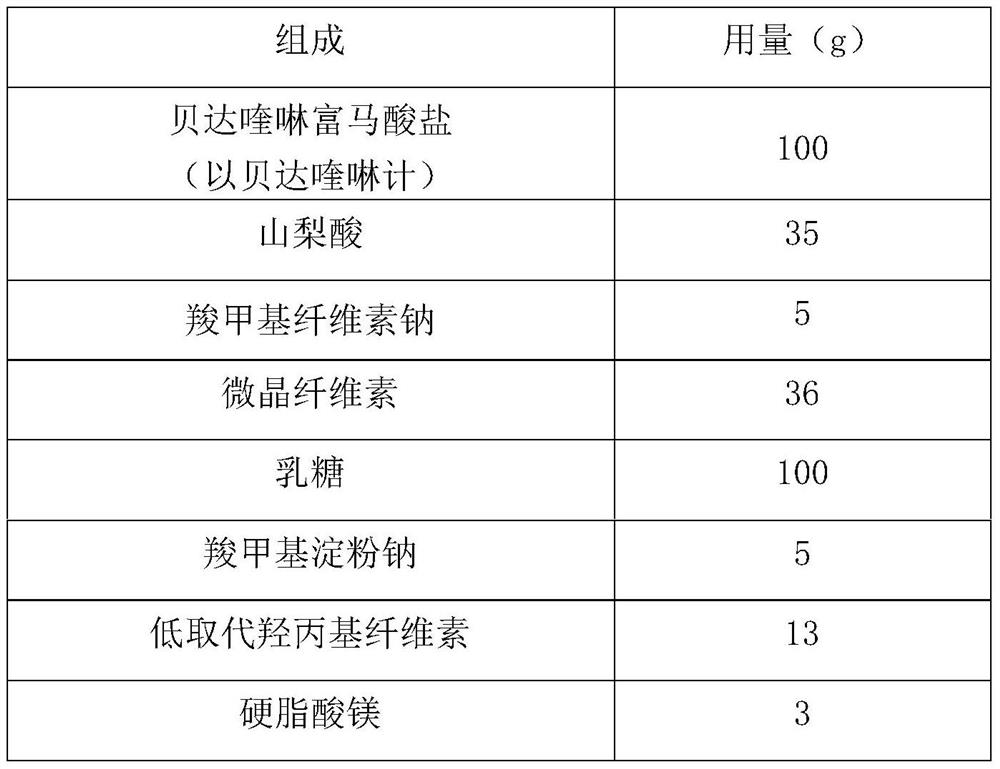
Embodiment 2
[0030]
[0031] Preparation:
[0032] (1) Preparation of bedaquiline solid composition:
[0033] (i) adding sorbic acid and sodium carboxymethyl cellulose to 240 mL of aqueous ethanol containing 50% ethanol and dissolving it into a mixed solution;
[0034] (ii) In the bedaquiline fumarate of 50 μm in the particle diameter d0.9 placed in the fluidized bed, spray the above mixed solution, granulate, dry, and granulate to obtain the bedaquiline solid composition , wherein, the weight ratio of particles with a particle size smaller than 150 μm in the solid composition is 63.6% (w / w).
[0035] (2) Preparation of bedaquiline preparation:
[0036] The bedaquiline pharmaceutical preparation is obtained by mixing the above-mentioned bedaquiline solid composition with microcrystalline cellulose, lactose, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and magnesium stearate, and pressing into tablets.
Embodiment 3
[0038]
[0039]
[0040] Preparation:
[0041] (1) Preparation of bedaquiline solid composition:
[0042] (i) adding malic acid and hydroxypropyl cellulose to 250 mL of ethanol aqueous solution containing 70% ethanol and dissolving to make a mixed solution;
[0043] (ii) In the bedaquiline fumarate of 46 μm in the particle diameter d0.9 placed in the fluidized bed, spray the above mixed solution, granulate, dry, and granulate to obtain the bedaquiline solid composition , wherein, the weight ratio of particles with a particle size smaller than 150 μm in the solid composition is 68.9% (w / w).
[0044] (2) Preparation of bedaquiline preparation:
[0045] The above-mentioned bedaquiline solid composition is mixed with microcrystalline cellulose, lactose, crospovidone, croscarmellose sodium and sodium stearyl fumarate to obtain the bedaquiline drug preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com