Synthesis method of 6-bromophthalazine
A synthetic method and phthalazine technology, which are applied in the field of synthesis of 6-bromophthalazine, can solve the problems of harsh reaction conditions, low reaction yield, unsuitable amplification, etc., and achieve mild reaction conditions, high yield, and improved yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
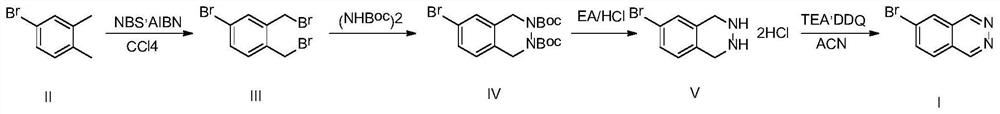
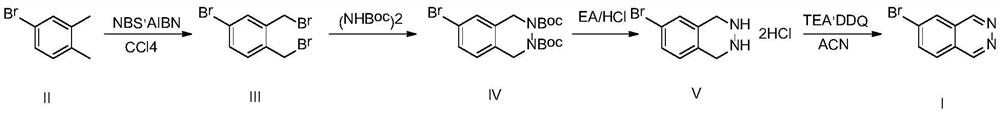
[0038] 4-Bromo-1,2-xylene (200g, 1.08mol, 1.0eq), NBS (500g, 2.81mol, 2.6eq) and AIBN (19.7g, 0.12mol, 0.11eq) were added to 2L of tetrachloride In carbon, the temperature was raised to reflux, reacted under this condition for 3 hours, cooled to room temperature, filtered, and the filtrate was spin-dried and then slurried with 400 mL of petroleum ether, filtered, and the solvent was removed under reduced pressure to obtain compound III (297 g, yield 80.1%);
Embodiment 2
[0040]
[0041] Compound III (280g, 0.82mol, 1.0eq) and tert-butyl hydrazinodicarboxylate (189.7 g, 0.82mol, 1.0eq) were dissolved in 2.8L of toluene, and a phase transfer catalyst TEAB (5.8g, 0.04 mol, 0.05eq) and 1.4L of 50% sodium hydroxide aqueous solution, then heated up to 100°C, reacted for 10 hours, after the reaction was completed, lowered to room temperature, separated, washed the organic phase with water, washed with saturated saline and dried, and then spin-dried , adding 500mL of methyl tert-butyl ether to the crude product, beating for 0.5 hours, filtering and drying to obtain compound IV (310g, yield 91.8%).
Embodiment 3
[0043]
[0044] Compound IV (300g, 0.73mol, 0.0eq) was dissolved in 1.5L of ethyl acetate, the temperature was lowered to 0°C, the temperature was controlled at 0-5°C, and 1.5L of ethyl acetate / HCl solution (4M) was added dropwise thereto , after the dropwise addition was completed, it was slowly raised to room temperature, reacted for 2 hours, and was filtered to obtain compound V (198 g, yield 95.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com