Therapeutic dendritic cell cancer vaccine and application thereof
A technology of megakaryocytes and shuttle plasmids, which is applied in the field of preparation of therapeutic cancer vaccines, can solve problems such as complex administration methods and potential safety hazards, and achieve the effect of simplifying culture time and experimental operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
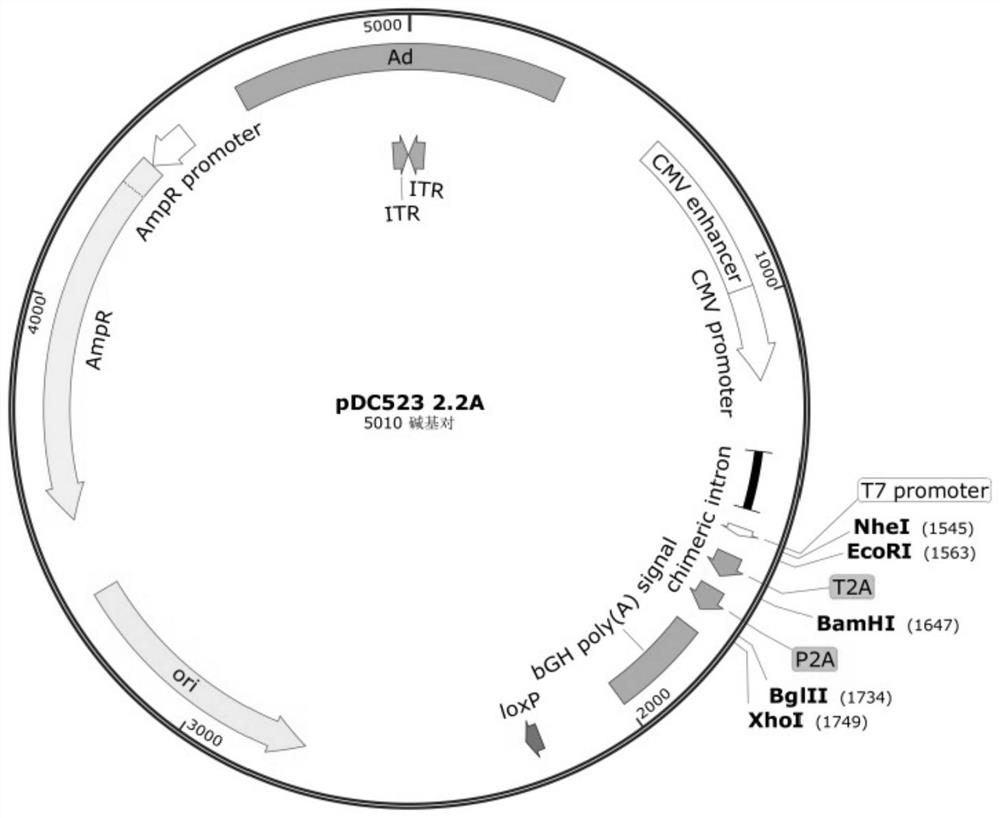
[0048] Embodiment 1, transformation of shuttle plasmid
[0049] This embodiment is to construct the adenoviral shuttle plasmid that is suitable for coding polygene, with AdMAX TM Based on the pDC511 shuttle plasmid (purchased from MicroBix, Canada) in the system, another shuttle plasmid pDC523 2×2A was constructed, which specifically included the following steps:
[0050] 1.1. Synthesize a pair of primers, the upstream primer is 5'-gcg cta gct agctca ata ttg gcc att agcc-3' (SEQ ID NO:1, 5' end introduces Nhe I site), the downstream primer is 5'-gaa gat cta taa ctt cgt ataatg tat gct ata cga agt tat cga tcg agc cat aga gcc c-3' (SEQ ID NO: 2, 5' end introduces BglII site and LoxP sequence), with pcDNA3.1(-)-myc- His B plasmid (Invitrogen TM ) as a template, perform PCR amplification, high-fidelity Q5 PCR enzyme (NEB) is used for amplification, the amplification conditions are 98°C 10s, 72°C 20s, 72°C 60s, 30 cycles, amplified to obtain a 1180bp PCR fragment (i.e. the CMVp-p...
Embodiment 2
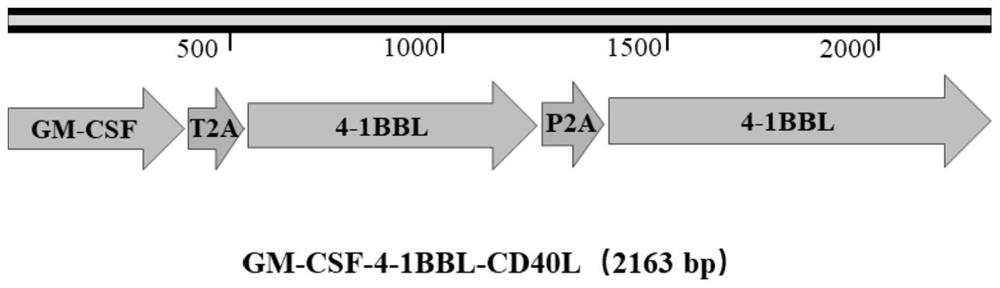
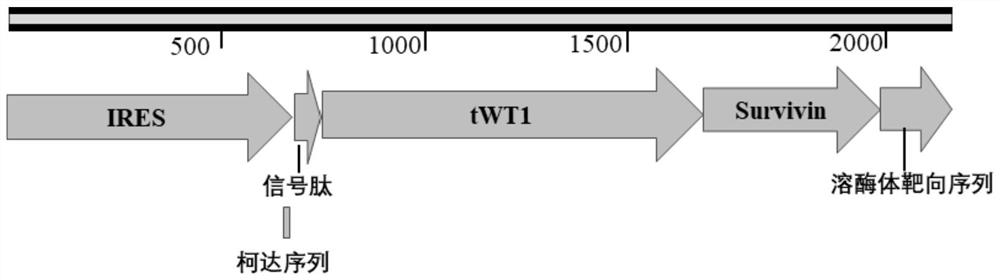
[0052] Cloning of embodiment 2, WT1, Survivin, GM-CSF, CD40L and 4-1BBL genes
[0053] This embodiment is to insert the target genes WT1, Survivin, GM-CSF, CD40L and 4-1BBL into the pDC523 2×2A plasmid obtained in the above-mentioned embodiment 1, specifically including the following steps:
[0054] 2.1. Take 5ml of peripheral blood from healthy people, separate peripheral blood mononuclear cells (PBMCs) with lymphocyte separation medium, stimulate with LPS (10ng / ml) for 12 hours, and then use RNA extraction kit (Qiagen) to extract the total RNA of the cells. Using RNA as a template, cDNA was synthesized with a reverse transcription kit (Takara) and named as PBMCs cDNA; 1×10 cDNA was extracted with an RNA extraction kit (Qiagen). 6 The total RNA of individual K562 leukemia cells was used as a template to synthesize cDNA with a reverse transcription kit (Takara), which was named K562 cDNA.
[0055] 2.2. In this step, a total of 6 pairs of primers were designed for amplifying t...
Embodiment 3
[0064] Example 3, Construction of a recombinant adenoviral vector capable of expressing multiple genes simultaneously
[0065] This example is based on AdMax TMCre recombinant adenovirus system instructions for the construction of recombinant adenoviruses expressing and encoding multiple genes. Extract the plasmid from the shuttle plasmid pGBC-IRES-WSL obtained in Example 2 above using a plasmid midi kit (EndoFreePlasmid Midi Kit, QIAGEN), and determine that the 260 / 280 ratio is around 1.9, and adjust the plasmid concentration to 1 μg / μl; pBGHfrt△ E1,3Cre (for the construction of type 5 adenovirus vector) (purchased from Microbix, Canada) and pBGHfrt△E1,3Cre(5 / F35) (for the construction of type 5 / F35 adenovirus vector, Beijing Benyuan Zhengyang Gene Co., Ltd. The adenovirus backbone plasmid (purchased by the company) was extracted with the Plasmid Maxi Kit (EndoFree Plasmid Maxi Kit, QIAGEN), and the 260 / 280 ratio was determined to be around 1.9, and the plasmid concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com