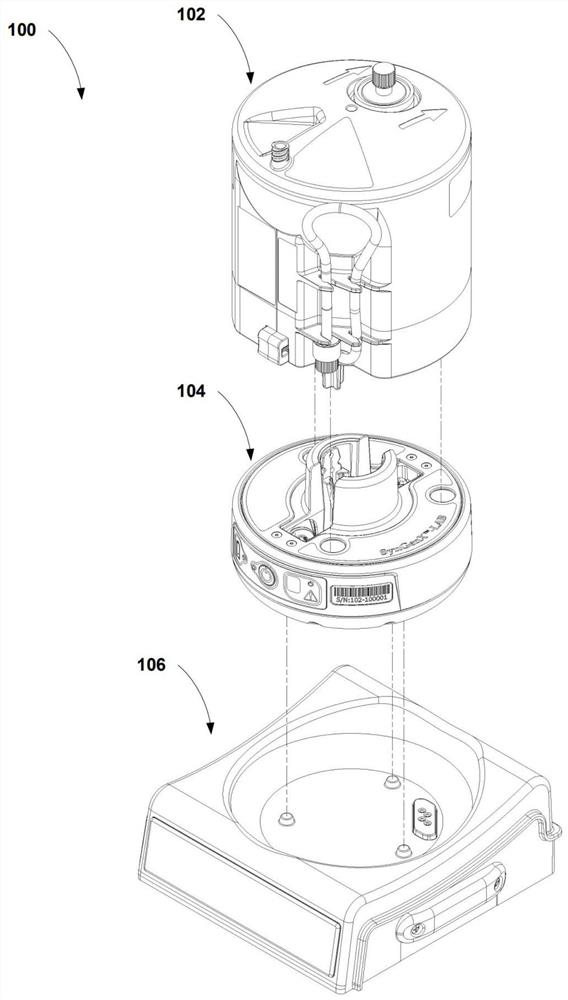
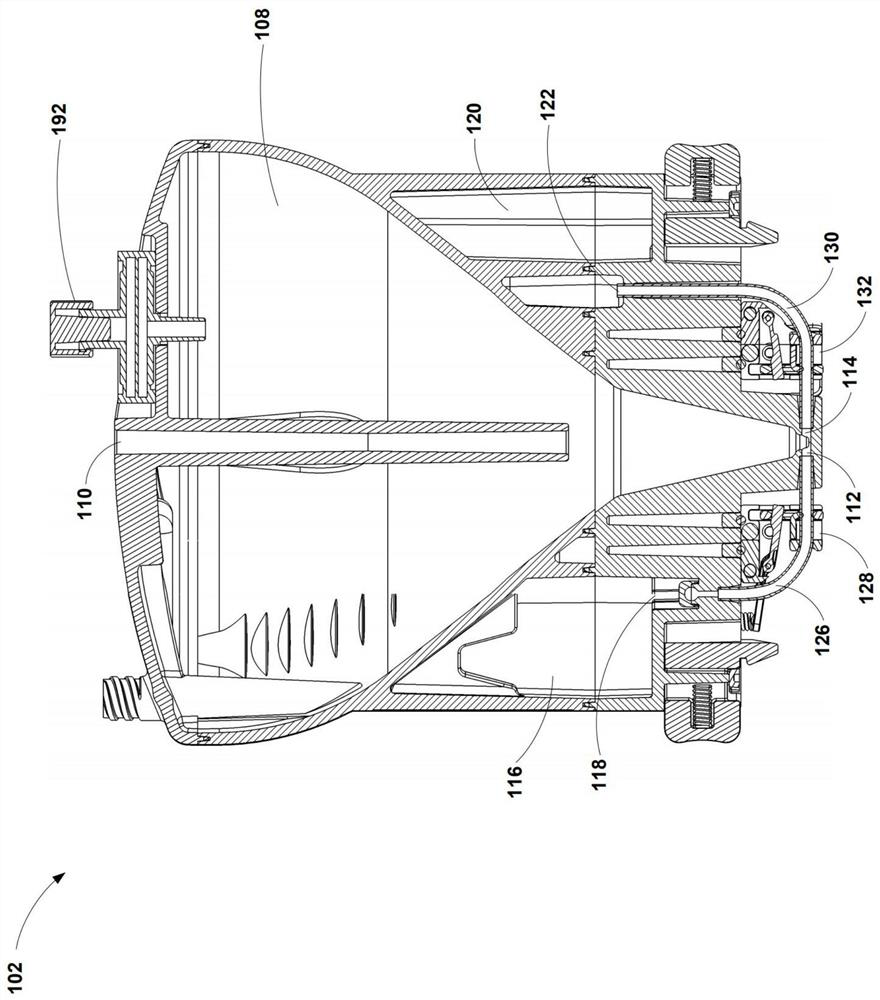
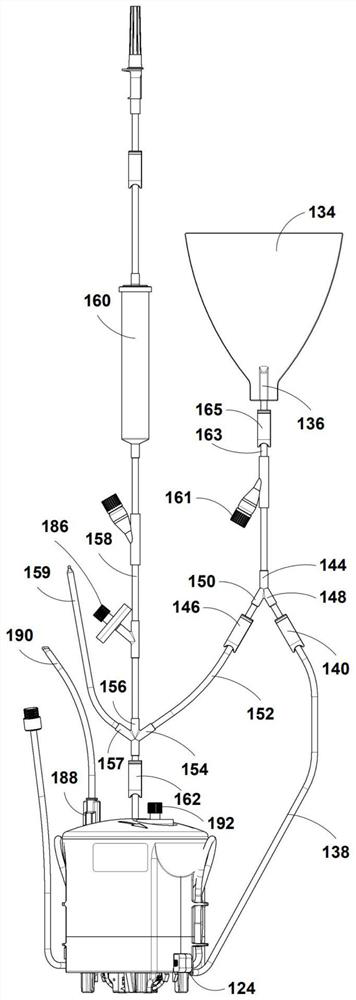
Buoyancy-activated cell sorting (BACS)-compatible activation/transduction systems and methods
A transduction and cell technology, applied in the direction of cell culture active agents, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of high cost of therapeutic agents, negative impact on cell proliferation ability, and complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Isolation and activation of T lymphocytes by microbubble / floating reagent
[0155] In order to compare CD3 isolated and activated by the method of the present invention or conventional methods + For T cell performance, the following protocol was used:
[0156] Isolation and activation of cells by CD3 / 28 BACS reagent:
[0157] 1. Healthy human peripheral blood mononuclear cell (PBMC) preparations are prepared by processing fresh blood in the cartridge of the '394 patent, centrifuged without Ficoll centrifugation. In short, it includes:
[0158] a. Put 170ml of peripheral blood into the main chamber of the sleeve, and put 10ml of Dulbecco's phosphate-buffered saline plus 1% human serum albumin and 2mM EDTA (DPBS-AE, referring to the mixture of the three) into the harvest chamber ( harvest chamber).
[0159] b. The blood was separated by centrifugation at 2000×g for 20 minutes, centrifuged at 50×g for 5 minutes to remove red blood cells, centrifuged at 1000×g for 5 min...
Embodiment 2
[0192] Microvesicles themselves do not activate T cells non-specifically
[0193] To test whether the activation of T cells by BACS reagents might be molecularly nonspecific (i.e., a function of the lipid-shelled air-core microvesicles themselves, irrespective of their binding antibodies to T cells), we compared the use of anti-CD3 / CD28 microvesicles and Isolation / activation of T cells using anti-CD4 / CD8 microvesicles. CD4 and CD8 are T cell surface antigens that are not involved in triggering T cell activation.
[0194] Healthy human T cells were isolated, activated, and expanded using BACS reagents (from a donor different from Example 1) as described in Example 1 for microvesicles associated with anti-CD3 / CD28 antibodies or with Isolation / activation steps of anti-CD4 / CD8 antibody-associated microvesicles. After 14 days in culture, T cells isolated with CD3 / CD28 microvesicles expanded 2247-fold, whereas T cells from the same donor isolated with CD4 / CD8 microvesicles expande...
Embodiment 3
[0197] Viral transduction of microvesicle / buoyancy reagent-activated T lymphocytes
[0198] activate CD3 + The purpose of T cells to generate CAR-T cells is to sensitize T cells to viral transduction of synthetic chimeric antigen receptor genes. In this example, CD3 isolated and activated by the method outlined in Example 1 was transduced with a viral vector + T cells were therefore transduced with a vector, in this case with the green fluorescent protein (GFP) gene, to confirm that BACS-activated cells were similar to those activated by conventional magnetic beads.
[0199] 1. As described in Example 1, adopt CD3 / CD28 BACS microvesicle or CD3 / CD28 Dynabeads to healthy human CD3 + T cells (from a different donor than in Examples 1 and 2) were isolated and activated. at 37°C and 5% CO 2 After 36 hours of incubation in a T25 flask under the conditions of 0.5 x 10 6 The density of cells / ml was inoculated onto cells coated with reverse junction protein (Retronectin, 10 μg / cm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com