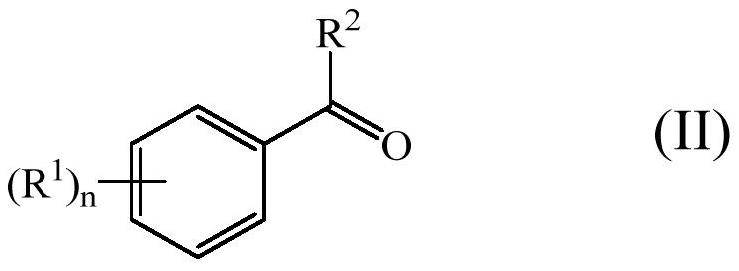
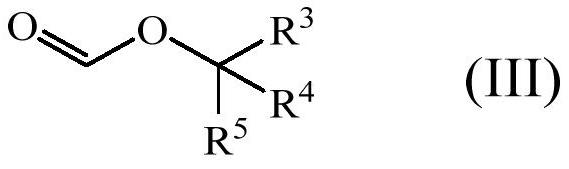
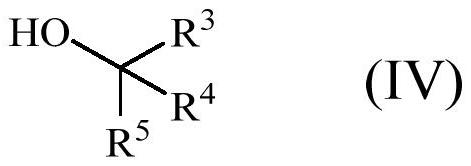Enol ether properfume
A compound, alkyl technology, applied in the field of formula compounds, which can solve the problems of non-retention, lack of persistence, not very strong experience, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Example 1. (1-(octyloxy)prop-1-en-2-yl)benzene: diphenylpropanal (hydratropaldehyde) (10g, 74.5mmol), octanol (24.3g, 186mmol), A mixture of TsOH (0.28 g, 1.49 mmol) and toluene (100 mL) was heated at reflux for 1 h while removing the water of reaction with a Dean-Stark trap. After the mixture was cooled, it was diluted with EtOAc and washed with saturated NaHCO 3 and washed with saturated NaCl. Na for organic phase 2 SO 4 Dry, filter and concentrate to give crude dioctyl acetal. About a quarter (19.3mmol) of the material was mixed with KHSO 4 (0.5 g, 3.67 mmol) were mixed and heated under vacuum (30 torr) using a Kugelrohr distillation apparatus. After 2.5 hours at 140°C and an additional 2.5 hours at 160°C, GC analysis indicated that most of the acetal had been converted to the enol ether. Kugelrohr distillation (145 °C, 80 mTorr) afforded 3.46 g of enol ether (14.0 mmol, 73% yield) as a colorless liquid (E / Z=81:19). 1 H NMR (CDCl 3 , 500MHz, E-isomer): δ0.88(...
Embodiment 2~7
[0162] Examples 2-7. Dimethyl acetal (4 g, 22.1 mmol), alcohol (66 mmol) and KHSO 4 (33 mg, 0.22 mmol) for 1-2 hours to achieve the exchange of the methoxy group with the alcohol while removing the released methanol. Thereafter, the temperature is increased (120-140° C.) and the pressure is decreased (25-50 mTorr) to complete the elimination reaction to form the enol ether while removing excess alcohol. The enol ether was then distilled off from the reaction mixture (160-180°C, 25-50 mTorr). The product can be further purified by a second Kugelrohr distillation if desired.
Embodiment 2
[0163] Example 2. (1-(((Z)-hex-3-en-1-yl)oxy)prop-1-en-2-yl)benzene: from (Z)-3-hexene-1- Starting from alcohol, the title compound was isolated as a colorless oil in 37% yield (E / Z=80:20).
[0164] 1 H NMR (CDCl 3 , 500MHz, E-isomer): δ0.97(t, J=7.5Hz, 3H), 1.99(d, J=1.3Hz, 3H), 2.07(pentet, J=7.3Hz, 2H), 2.41( q,J=7.0Hz,2H),3.83(t,J=7.0Hz,2H),5.33-5.40(m,1H),5.47-5.54(m,1H),6.46(q,J=1.3Hz,1H ),7.12-7.17(m,1H),7.24-7.32(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com