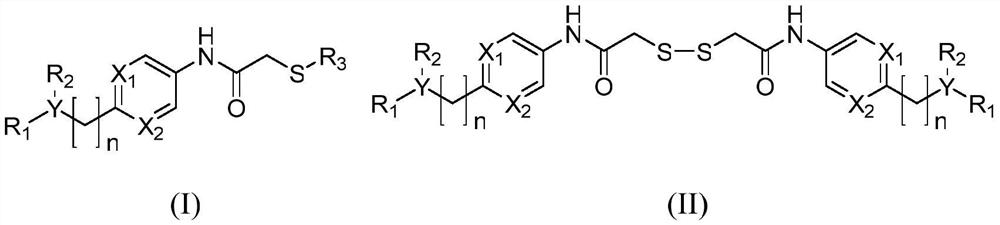
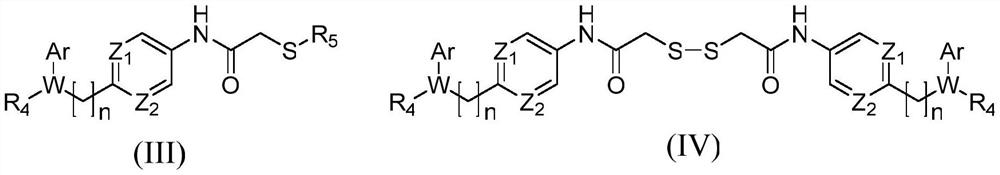
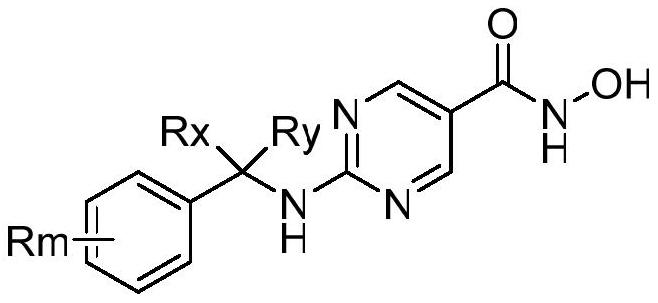
Histone deacetylase subtype inhibitor thioacetyl arylamine compound and application thereof
A technology of acetylase subtype and agent sulfur acetylarylamine, which is applied in the application field of treating tumors or neurodegenerative diseases, and can solve problems such as low bioavailability, genotoxicity, and poor pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Preparation of 2-mercapto-N-(2-(methyl(phenyl)amino)pyrimidin-5-yl)acetamide (1)
[0112]
[0113] Prepared according to the general method of synthesis, the synthetic route is as follows:
[0114]
[0115] Reagents and conditions: (a) HBTU, DIPEA, THF, RT, overnight; (b) TFAA, CH2Cl2, RT, 3h; (c) K2CO3, DMF, 120℃, 6h; (d) H2, Pd / C, RT ,6h.
[0116] Synthesis of N-methyl-5-nitro-N-phenylpyrimidin-2-amine (1-2)
[0117] In a 250ml three-necked flask, add 2-chloro-5-nitropyrimidine (8g, 50.15mmol), potassium carbonate (2eq), N-methylanilinamine (1.1eq) successively, DMF is used as solvent, and under nitrogen protection, 140 ℃ reaction 6h. After cooling to room temperature, the mixture was quenched with ice-water, extracted twice with ethyl acetate, and the organic phase was dried and separated by column chromatography to obtain 8.4 g of a yellow solid with a yield of 72.76%. 1H NMR(600MHz,DMSO-d6)δ:3.58(s,3H),7.31–7.37(m,1H),7.37–7.43(m,2H), 7.47(t,J=...
Embodiment 2
[0124] Example 2 Preparation of 2-mercapto-N-(6-(methyl(phenyl)amino)pyridin-3-yl)acetamide (2)
[0125] 2 was prepared according to the general method.
[0126] 1 H NMR (600MHz, Chloroform-d) δ8.68 (d, J = 1.5Hz, 1H), 7.63 (dd, J = 7.4, 1.5Hz, 1H), 7.37–7.30 (m, 2H), 7.26–7.20 ( m,2H),7.12–7.03(m,2H),3.58(d,J=7.0Hz,2H), 3.45(s,2H),2.85(t,J=7.0Hz,1H), MS(ESI)m / z:274.1(M+).
Embodiment 3
[0127] Example 3 Preparation of 2-mercapto-N-(4-(methyl(phenyl)amino)phenyl)acetamide (3)
[0128] 3 was prepared according to the general method.
[0129] 1 H NMR (600MHz, Chloroform-d) δ: 9.88(s,1H), 7.76–7.70(m,2H), 7.28–7.20(m,2H), 7.12–7.04(m,3H), 6.72–6.66(m , 2H), 3.62 (d, J=6.9Hz, 2H), 3.37(s, 2H), 2.85(t, J=6.9Hz, 1H), MS (ESI) m / z: 273.1 (M+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com