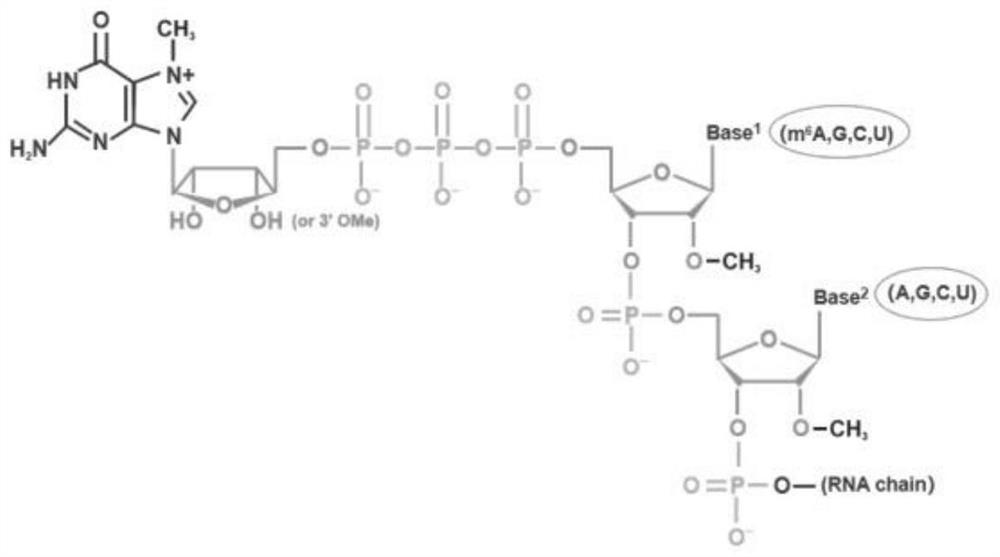
Novel Cap2 structure 5' cap analogue and preparation method thereof
A technology of analogs and caps, which is applied in the field of genetic engineering, can solve the problems of high immunogenicity, low mRNA yield, and low capping rate, and achieve the effects of high synthesis efficiency, low immunogenicity, and high capping efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] The present invention also provides a preparation method of the novel Cap2 structure 5' cap analog described in the above technical scheme, comprising the following steps:
[0098] 1) Dissolve 2'-O-Methyl-ATP, 2'-O-Methyl-GTP, 2'-O-Methyl-CTP and 2'-O-Methyl-UTP in RNase-free water respectively, and then mix with Phosphohydrolase and 2×Reaction Buffer were mixed and incubated to obtain 2′-O-Methyl-ADP, 2′-O-Methyl-GDP, 2′-O-Methyl-CDP and 2′-O-Methyl-UDP;
[0099] 2) 2'-O-Methyl-ADP, 2'-O-Methyl-GDP, 2'-O-Methyl-CDP and 2'-O-Methyl-UDP obtained in step 1) were dissolved in RNA-free Mixed with 7-Methylguanosine, 7-Methyl-3′-O-Methylguanosine, guanosyltransferase, and 2×Reaction Buffer in enzyme water for incubation to obtain m7G(5′)ppp(5′)(2′OMeA / G / C / U) and 3'-O-Me-m7G(5')ppp(5')(2'OMeA / G / C / U);
[0100] 3) m7G(5')ppp(5')(2'OMeA / G / C / U) and 3'-O-Me-m7G(5')ppp(5')( 2′OMeA / G / C / U) were dissolved in RNase-free water, mixed with T4 RNA Ligase1, and then mixed with 2′-O-Meth...
Embodiment 1
[0110] 1. Dissolve 10mmol of 2'-O-Methyl-ATP, 2'-O-Methyl-GTP, 2'-O-Methyl-CTP or 2'-O-Methyl-UTP in RNase-free water, total The volume is 14 μl. Add 1μl (50000U) phosphohydrolase and 15μl 2×ReactionBuffer (50mM Tris-HCl; 5mM KCl; 1mM MgCl 2 ; 1mM DTT; pH 8), incubated at 37°C for 1h to obtain 2′-O-Methyl-ADP, 2′-O-Methyl-GDP, 2′-O-Methyl-CDP and 2′-O-Methyl-UDP .
[0111] 2. Purify the 2'-O-Methyl-ADP, 2'-O-Methyl-GDP, 2'-O-Methyl-CDP and 2'-O-Methyl-UDP obtained above by HPLC and dissolve them in RNA-free Enzyme water, total volume 11 μl.
[0112] 3. Add 10 μl (10mmol) 7-Methylguanosine, 10 μl (10mmol) 7-Methyl-3′-O-Methylguanosine and 1 μl (50000U) guanosine transferase to the solution obtained in step 2, add 22 μl 2×ReactionBuffer (50mM Tris-HCl; 5mM KCl; 1mM MgCl 2 ; 1mM DTT; pH 8), incubated at 37°C for 1h to obtain m7G(5′)ppp(5′)(2′OMeA / G / C / U) or 3′-O-Me-m7G(5′) ppp(5')(2'OMeA / G / C / U).
[0113] 4. The solution obtained in step 3 was purified by HPLC and dissolved ...
Embodiment 2
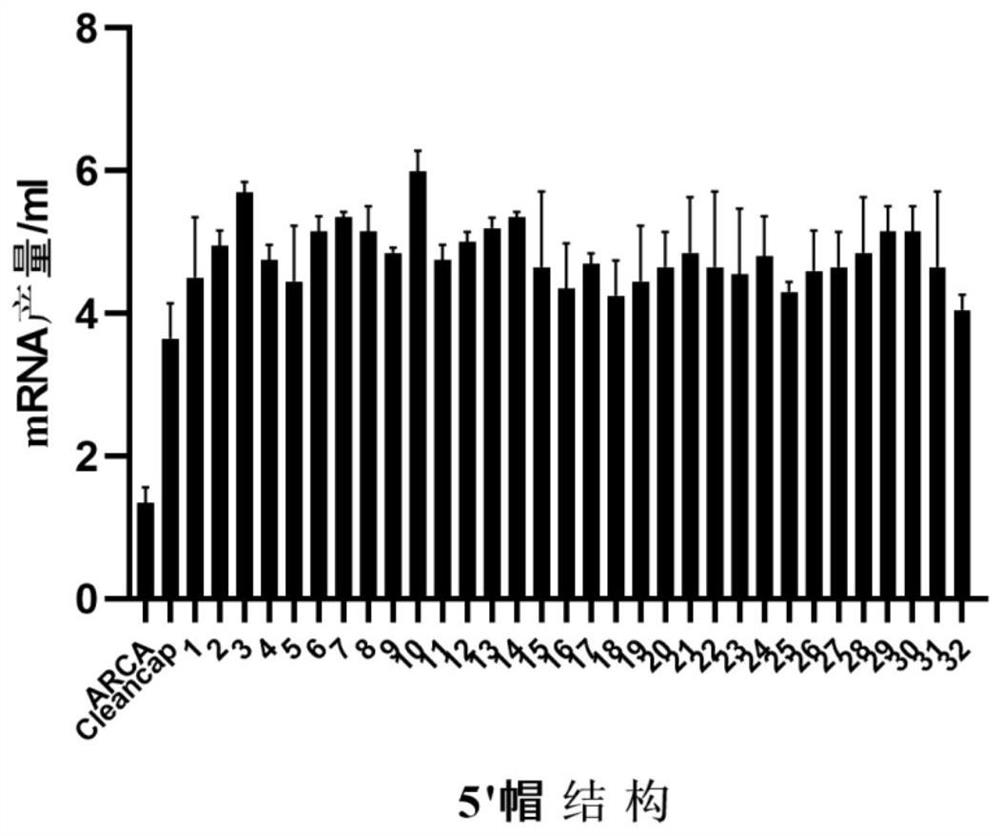
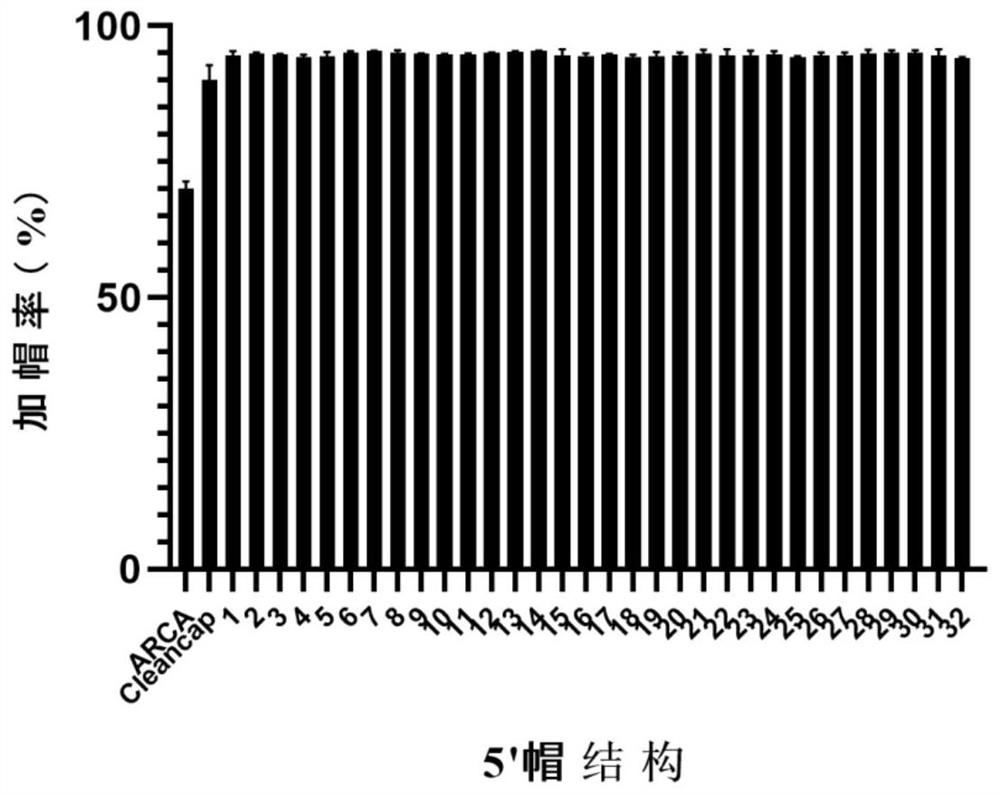
[0148] The 32 novel Cap2 cap structure analogs prepared in Example 1 were applied to mRNA synthesis to effectively increase the synthesis efficiency of mRNA (4-6 mg / ml).
[0149] experimental method:
[0150] 1. Before synthesizing mRNA, linearize the plasmid with NotI and digest overnight at 4°C.
[0151] 2. DNA template extraction.
[0152] 3. In vitro transcription and synthesis of mRNA, respectively using ARCA, Cleancap and 32 kinds of Cap2 in the present invention as the cap structure, the reaction system is shown in Table 1:
[0153] Table 1 Reaction system
[0154] components Dosage T7 10X Reaction Buffer 2ul T7 ATP Solution(75mM) 2ul T7 CTP Solution(75mM) 2ul T7 GTP Solution(75mM) 2ul T7 UTP Solution(75mM) 2ul Linearized plasmid template <8ul
T7 Enzyme Mix 2ul ARCA / Cleancap / Cap2 2ul Nuclease-free water up to 20ul
[0155] React at 37°C for 6 hours. TURBO DNase digestion for 15 minutes.
[015...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com