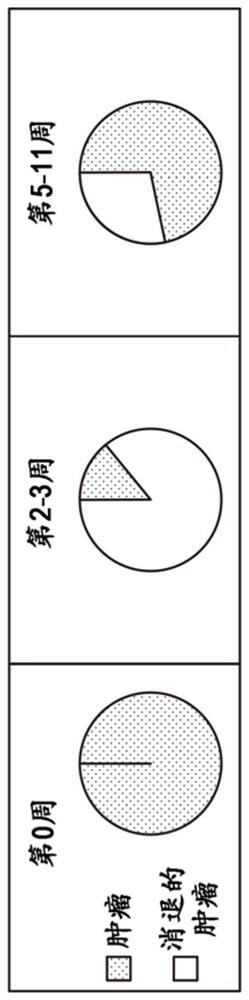
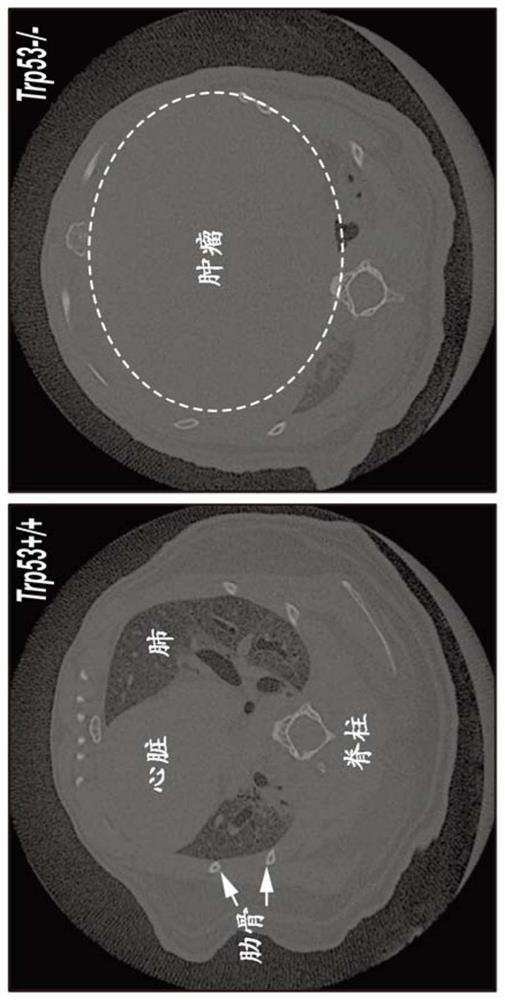
Combination therapies for treating cancers
A cancer, complex technology used in the field of combination therapy for the treatment of cancer, capable of addressing DNA damage, damage to non-malignant cells, mutations or breaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] In some embodiments, the composition is a pharmaceutical composition. Pharmaceutical compositions are combinations of active agents (such as glycolysis inhibitors and / or RAD51 complex inhibitors) with carriers (inert or active), making the compositions especially suitable for in vivo or ex vivo therapeutic use. A pharmaceutically acceptable carrier does not cause undesired physiological effects upon or when administered to a subject. The carrier in the pharmaceutical composition must also be acceptable in the sense of being compatible with and capable of stabilizing the active agent. One or more solubilizing agents can be used as a drug carrier to deliver the active agent. Examples of pharmaceutically acceptable carriers include, but are not limited to, biocompatible vehicles, adjuvants, additives, and diluents to obtain compositions that can be used as dosage forms. Examples of other carriers include colloidal silicon oxide, magnesium stearate, cellulose and sodium l...
Embodiment 1
[0072] Example 1: 2DG Reduces Tumor Burden in a Spontaneous Mouse Model of Lymphomagenesis SJL / J Mice Spontaneously Develop a Proliferative Disease Involving CD4+ T and B Cells Similar to Non-Hodgkin's Lymphoma And it is evident after one year of age (27, 28). It is thought that in this model, activated CD4 that secretes interleukin-21 + T cells drive B cell transformation (29). CD8a-deficient, and thus CD8+ deficient, SJL / J mice exhibit a markedly accelerated development of B-cell lymphoma without other changes in their phenotype (30). As any tumor requires energy for growth or maintenance, highly proliferative cells, such as cancer cells, rely on multiple means of ATP production, including glycolysis, to meet their energy needs (4), thus following cellular glucose uptake Blocking glycolysis in cancer cells as a first step should theoretically reduce tumor burden (6, 7).
[0073] To test the extent to which glycolytic inhibition by 2DG attenuates these spontaneously arisi...
Embodiment 2
[0078] Example 2: Lung Tumor PDX Models Show that Metabolic Differences, Not Proliferation, Determine Susceptibility to 2DG
[0079] The effect of 2DG was evaluated in a PDX (patient-derived xenograft) model of human lung cancer. Despite the understanding that 2DG primarily affects glycolysis, studies have shown that 2DG may interfere with other systems, such as the cell cycle, independently of its effect on metabolism (8, 32, 33). Changes in the cell cycle will affect the proliferation of cancer cells and ultimately tumor growth. To test whether 2DG affects glycolysis independently of proliferation, two species with similar growth kinetics ( Figure 2A ) but the glycolytic pathway used differently by PDX human lung cancers (TM00244 and TM00921) (Table 1).
[0080] Table 1: Pathway differences between TM00921 and TM00244 (INGENUITY / KEGG pathway)
[0081]
[0082]
[0083] A direct comparison of global gene expression between the two cancers revealed that one cancer, TM0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com