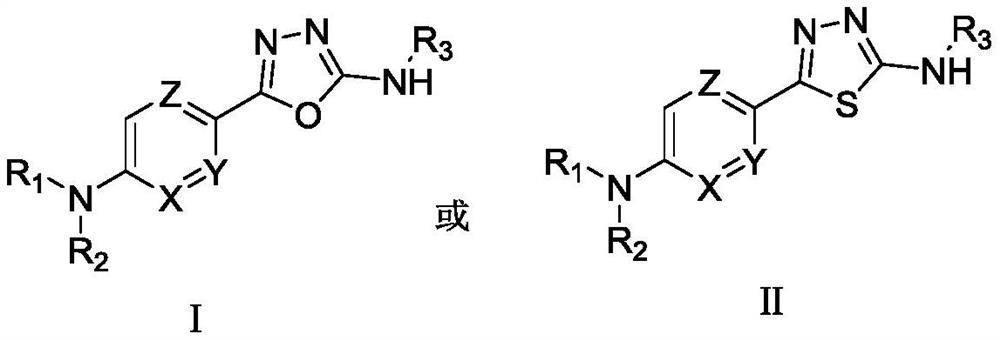
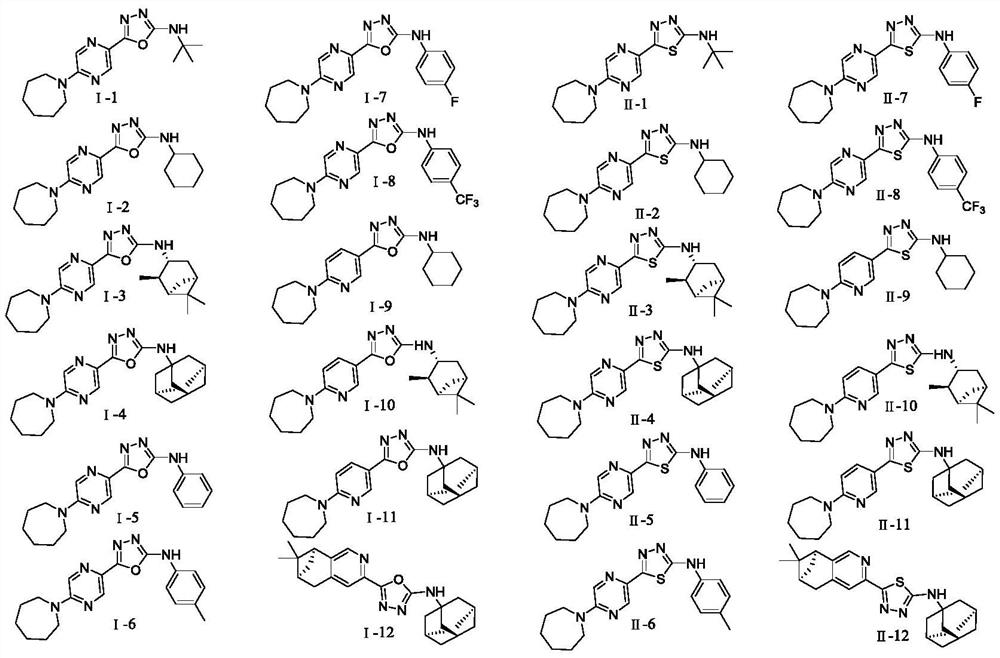
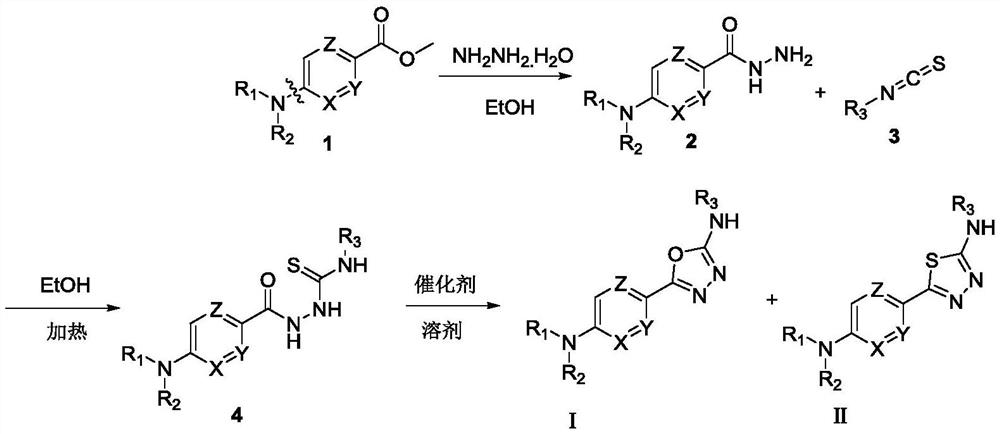
5-Aryl-1,3,4-thiodiazole/1,3,4-oxadiazole-2-amine compound and preparation method and application thereof
A technology of amine compounds and thiadiazoles, applied in the field of medicine, can solve the problems such as no literature reports, and achieve the effects of good inhibitory activity, high yield and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 2-(5-(cyclohexaneimin-1-yl)pyrazin-2-yl)-5-tert-butyl-1,3,4-oxadiazole (Ⅰ-1) and 2-(5-( Synthesis of cyclohexylimin-1-yl)pyrazin-2-yl)-5-tert-butyl-1,3,4-thiadiazole (Ⅱ-1)
[0037]
[0038] 1) Using methyl 5-chloropyrazine-2-carboxylate as a raw material, obtain 5-(cyclohexylimino-1-yl)pyrazine-2-hydrazide through hydrazinolysis reaction: take a 100mL round-bottomed flask, place in At 0°C, methyl 5-chloropyrazine-2-carboxylate (5.18g, 30mmol), CH 3CN (18mL), cyclohexylimine (3.27g, 33mmol, 3.7mL) were stirred evenly, and then DIPEA (10mL) was added dropwise. After the addition was complete, the reaction was stirred at 85°C and refluxed for 10h. Concentrate by rotary evaporation under reduced pressure, dissolve with dichloromethane (200mL), wash with saturated sodium bicarbonate solution and saturated brine successively, dry the organic phase with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain compound (4.5g). Proceed to the next ...
Embodiment 2
[0046] 2-(5-(cyclohexaneimin-1-yl)pyrazin-2-yl)-5-tert-butyl-1,3,4-oxadiazole (Ⅰ-2) and 2-(5-( Synthesis of Cyclohexylimin-1-yl)pyrazin-2-yl)-5-tert-butyl-1,3,4-thiadiazole (Ⅱ-2)
[0047]
[0048] 1) Using methyl 5-chloropyrazine-2-carboxylate as a raw material, obtain 5-(cyclohexylimino-1-yl)pyrazine-2-hydrazide through hydrazinolysis reaction: same as step 1 in Example 1 ).
[0049] 2) 5-(cyclohexylimino-1-yl)pyrazine-2-hydrazide reacts with cyclohexyl isothiocyanate to generate amidothiourea compound: take a 25mL round bottom flask and add the compound obtained in the previous step ( 300mg, 1.28mmol), cyclohexyl isothiocyanate (198.1mg, 1.4mmol) and absolute ethanol 5mL. After the addition was complete, the reaction was heated to 80° C., and the reaction was completed in 4 hours. Slowly cooled to room temperature, and then placed in the refrigerator for 4 hours, the solid precipitated, filtered, washed with cold ethanol, and dried to obtain 378 mg of cyclohexyl amidot...
Embodiment 3
[0057] 5-(5-(Cyclohexylimin-1-yl)pyrazin-2-yl)-N-((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1. 1] Hept-3-yl)-1,3,4-oxadiazol-2-amine (Ⅰ-3) and 5-(5-(cyclohexylimin-1-yl)pyrazin-2-yl) -N-((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-3-yl)-1,3,4-thiadiazol-2-amine Synthesis of (Ⅱ-3)
[0058]
[0059] In embodiment 1, step 2) tert-butyl isothiocyanate is replaced with pinanyl-3-isothiocyanate, and other operations are the same as in embodiment 1. 5-(5-(Cyclohexylimin-1-yl)pyrazin-2-yl)-N-((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1 .1] Hept-3-yl)-1,3,4-oxadiazol-2-amine (I-3) and 5-(5-(cyclohexylimin-1-yl)pyrazin-2-yl )-N-((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-3-yl)-1,3,4-thiadiazole-2- Amine (II-3) was purified by column chromatography to obtain compounds I-3 and II-3 (10 mg, yield 83.3%) and (140 mg, yield 85.3%), respectively.
[0060] Wherein the synthesis process of pinanyl-3-isothiocyanate is as follows: take a 250mL round bottom flask, add pinaneamine (4.5g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com