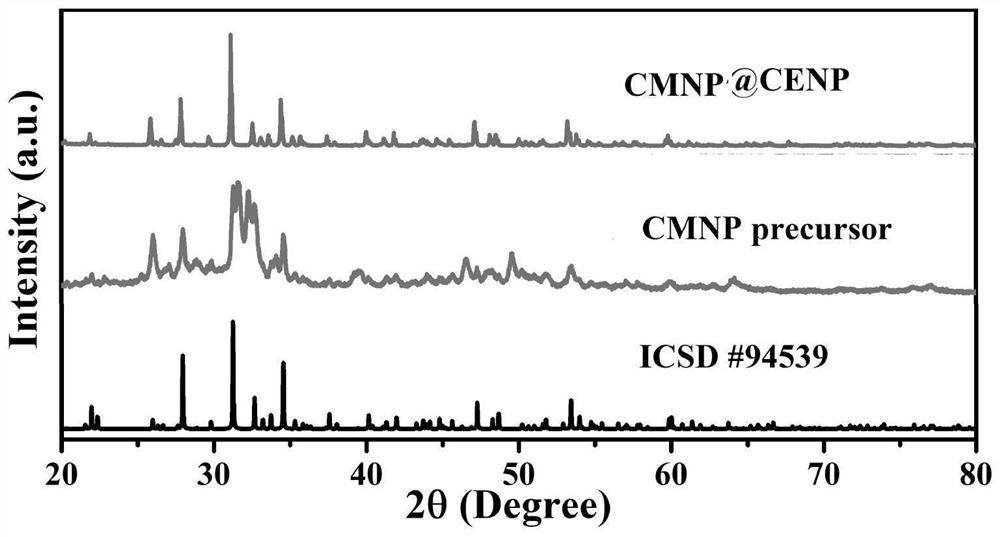
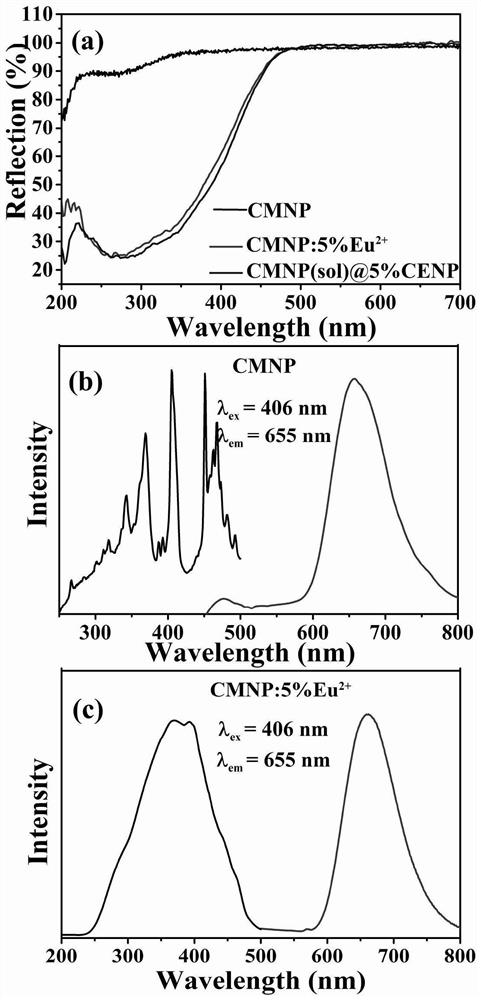
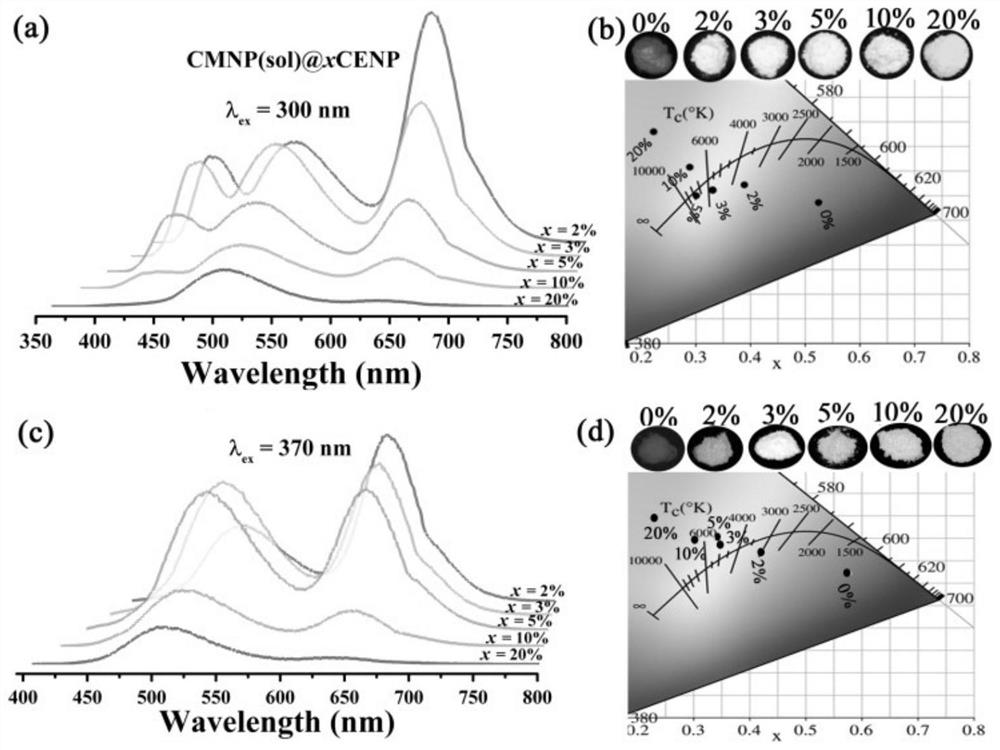
Preparation method of phosphate luminescent material with adjustable luminescent color and luminescent material prepared by the method
A technology of luminescent colors and luminescent materials, applied in luminescent materials, chemical instruments and methods, sustainable buildings, etc., can solve problems such as differences in thermal stability of phosphors, and achieve the effect of simple method, easy operation and good material performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: x=2%, CMNP(sol)@2%CENP fluorescent material of yellow light emission
[0056] (1) Synthesis of 1mmol CMNP(sol)
[0057] 2.1253gCa(NO 3 ) 2 • 4H 2 O, 0.0850gNaNO 3 , 0.2451gMn(CH 3 COO) 2 • 4H 2 O was dissolved in deionized water, added 4.6231g of citric acid monohydrate, and stirred to dissolve.
[0058] Add 0.8052 g (NH 4 )H 2 PO 4 , join HNO 3 Adjust the pH value to 3, add deionized water until the total volume of the solution is 50ml, add 5g of PEG, and stir for 2 hours to form a uniform wet sol.
[0059] at 80 o C in a water bath and heated until the xerogel is formed; finally, the xerogel was heated at 500 o The CMNP precursor (CMNP(sol)) was obtained after calcination in C air atmosphere for 4 hours.
[0060] (2) Preparation of CMNP(sol)@2%CENP samples
[0061] 0.0425gCa(NO 3 ) 2 • 4H 2 O, 0.0017gNaNO 3 , 2 mlEu(NO 3 ) 3 The solution (concentration: 0.01mol / L) was dissolved in deionized water, 0.0925g of citric acid monohydrate w...
Embodiment 2
[0064] Example 2: x=5%, CMNP(sol)@5%CENP fluorescent material emitting white light
[0065] (1) Synthesis of 1mmol CMNP(sol)
[0066] 2.1253gCa(NO 3 ) 2 • 4H 2 O, 0.0850gNaNO 3 , 0.2451gMn(CH 3 COO) 2 • 4H 2 O was dissolved in deionized water, added 4.6231g of citric acid monohydrate, and stirred to dissolve.
[0067] Add 0.8052 g (NH 4 )H 2 PO 4 , join HNO 3 Adjust the pH value to 3, add deionized water until the total volume of the solution is 50ml, add 5g of PEG, and stir for 2 hours to form a uniform wet sol.
[0068] at 80 o C water bath heated to xerogel formation; xerogel at 500 o The CMNP precursor (CMNP(sol)) was obtained after calcination in C air atmosphere for 4 hours.
[0069] (2) Preparation of CMNP(sol)@5%CENP samples
[0070] 0.1063gCa(NO 3 ) 2 • 4H 2 O, 0.0042gNaNO 3 , 5mlEu(NO 3 ) 3 The solution (concentration: 0.01mol / L) was dissolved in deionized water, 0.2312g of citric acid monohydrate was added, and stirred to dissolve.
[0071] Add...
Embodiment 3
[0073] Example 3: x=20%, CMNP(sol)@20%CENP fluorescent material emitting green light
[0074] (1) Synthesis of 1mmol CMNP(sol)
[0075] 2.1253gCa(NO 3 ) 2 • 4H 2 O, 0.0850gNaNO 3 , 0.2451gMn(CH 3 COO) 2 • 4H 2 O was dissolved in deionized water, added 4.6231g of citric acid monohydrate, and stirred to dissolve.
[0076] Add 0.8052 g (NH 4 )H 2 PO 4 , join HNO 3 Adjust the pH value to 3, add deionized water until the total volume of the solution is 50ml, add 5g of PEG, and stir for 2 hours to form a uniform wet sol.
[0077] at 80 o C water bath heated to xerogel formation; xerogel at 500 o The CMNP precursor (CMNP(sol)) was obtained after calcination in C air atmosphere for 4 hours.
[0078] (2) Preparation of CMNP(ol)@20%CENP samples
[0079] 0.4251Ca(NO 3 ) 2 • 4H 2 O, 0.0170gNaNO 3 , 20ml Eu(NO 3 ) 3 The solution (concentration: 0.01mol / L) was dissolved in deionized water, 0.9246g of citric acid monohydrate was added, and stirred to dissolve.
[0080] Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com