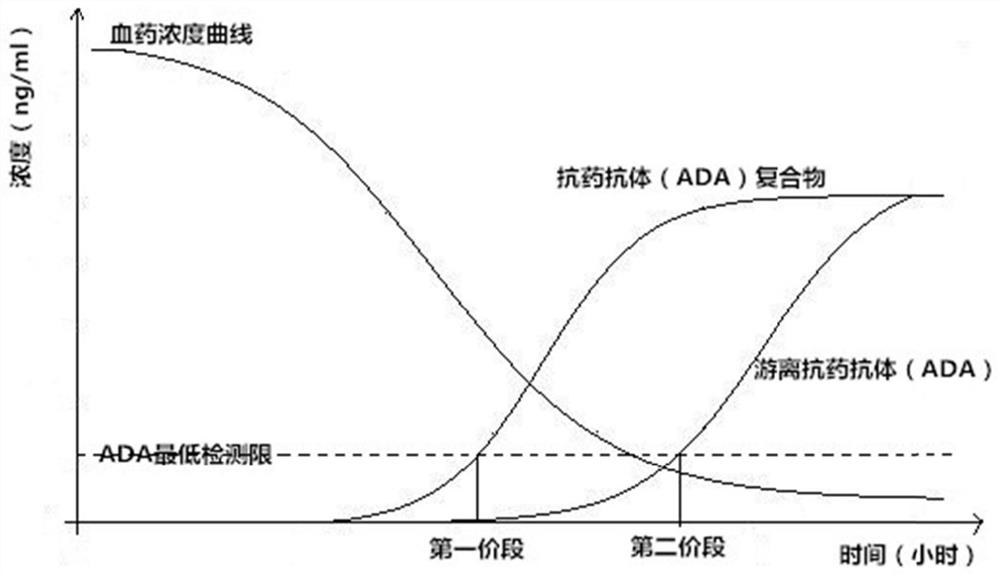
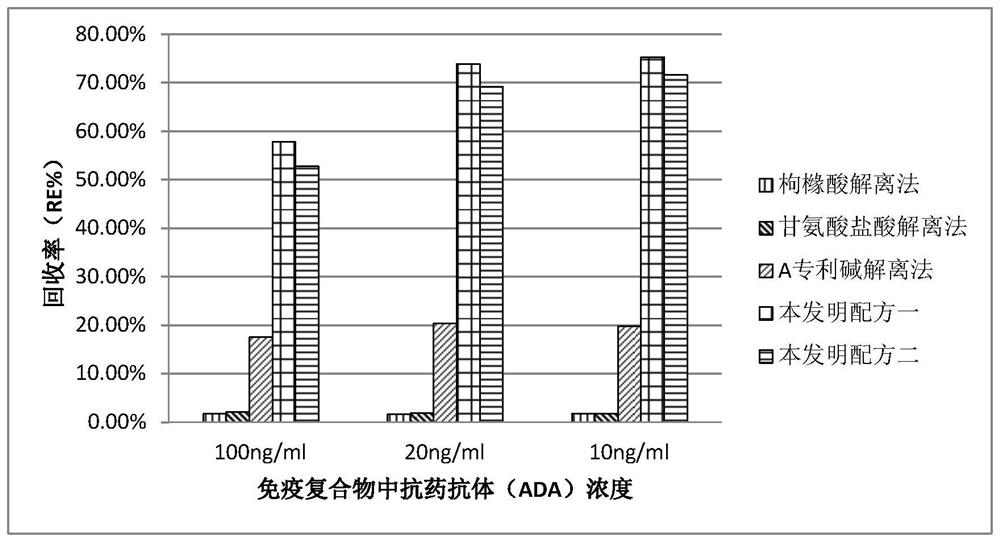
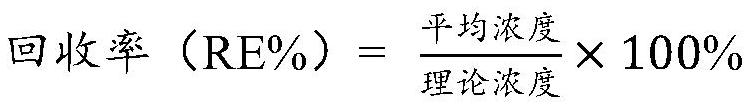Dissociation liquid for dissociating drug/ADA compound and application of dissociation liquid
A dissociation solution and complex technology, applied in the field of dissociation of antigen-antibody complexes, can solve problems such as complicated operation, intense composition, and complex components of the dissociation solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Preparation of the immune complex of immunoglobulin drug antibody 1 and anti-drug antibody 1
[0122] Preparation of samples to be tested:
[0123] According to the change of the drug in the human body after administration and the law of anti-drug antibody production, simulate the situation in the body, and prepare the immune complex with normal human pure serum: take antibody 1 and dilute it to 16 μg / mL with normal human pure serum; Drug antibody ADA1 (AbDSerotec, HCA204), was diluted with normal human pure serum to samples with a concentration of 200ng / mL, 140ng / mL, 40ng / mL and 20ng / mL, and then mixed with 16μg / mL of antibody 1 in equal volume respectively, 37 Incubate at ℃ for 1 hour to allow it to fully react and form immune complexes. In the above binding reaction between immunoglobulin and its antibody, the amount of immunoglobulin antibody 1 can completely bind to the antibody and is excessive. Prepare the test sample containing immune complex 1 (i.e...
Embodiment 2
[0124] Example 2: Study of Dissociated Components
[0125] (1) Detection method
[0126] Use direct ELISA to detect anti-drug antibodies in serum or plasma samples, with standard samples prepared from normal human pure serum and quality control samples with high (HQC), medium (MQC), and low (LQC) concentrations. Two replicate wells were set up for each concentration for the investigation of sensitivity, precision, accuracy and stability during detection and the evaluation of standard curve during sample determination. Use the four-parameter method of SoftMaxPRO software for curve fitting, and draw a graph on the log concentration (X)-absorbance (Y) coordinates to obtain the four parameters of the standard serum sample concentration (ie, the concentration of the anti-drug antibody standard in the sample) and absorbance The conversion equation is used to calculate the anti-drug antibody content in the serum or plasma sample to be tested on the same plate, and the average of 2 r...
Embodiment 3
[0146] Preparation of dissociation solution:
[0147] Option 1: Using water for injection as a solvent, add 0.5% sodium lauryl sulfate, 0.1% Tween-20, and 0.03% Triton X-100 in the dissociation solution, and the dissociation solution The pH is 6.4.
[0148] Scheme 2: using water for injection as a solvent, adding sodium lauryl sulfate with a mass concentration of 0.5% in the dissociation solution, and the pH of the dissociation solution is 7.4.
[0149] Preparation of samples to be tested: each sample to be tested was prepared with reference to Example 1, the difference being that the immunoglobulin drugs were replaced by Antibody 1, Antibody 2, and Antibody 3 respectively, and the anti-drug antibodies were anti-drug antibodies ADA1 (AbDSerotec, HCA204), anti-drug antibody ADA2 (AbDSerotec, HCA185), anti-drug antibody ADA3 (AbDSerotec, HCA256).
[0150] Dissociation and detection: In this example, the samples to be tested containing immunoglobulin drug antibody 1, antibody 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com