Synthetic method of difluoroacetyl fluoride
A technology of difluoroacetyl fluoride and synthesis method, which is applied in the field of synthesis of difluoroacetyl fluoride, can solve the problems of harsh fluorination reaction conditions, difficult operation, and high cost, and achieve the advantages of simple operation, lower reaction temperature, and simplified production process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of chromium complex catalyst among the present invention can refer to following documents and books:
[0035] (1) Efficient, Single-Step Access to Imidazo[1,5-a]pyridine N-Heterocyclic Carbene Precursors[J].ORGANIC LETTERS.2011Vol.13,No.19 5256–5259;
[0036] (2)(C ∧ C * )-cyclometalated platinum(II)imidazo[1,5-a]pyridine NHC complexes-Synthesis and characterization[J].Journal of Organometallic Chemistry.775(2015).155-163;
[0037] (3) Efficient synthesis of bulky N-Heterocyclic carbene ligands forcoinage metal complexes[J].Journal of Organometallic Chemistry.820(2016).1-7;
[0038] (4)Synthesis and characterization of novel cyclopentadienylmolybdenum imidazo[1,5-a]pyridine-3-ylidene complexes and their applicationin olefin oxidation catalysis[J].Journal of Catalysis.319(2014).119–126;
[0039] (5) Chiral imidazo[1,5-a]tetrahydroquinoline N-heterocyclic carbenes and their copper complexes for asymmetric catalysis[J].Tetrahedron:Asymmetry.24(201...
Embodiment 1
[0042]The concrete steps of the synthetic method of the difluoroacetyl fluoride of present embodiment are:
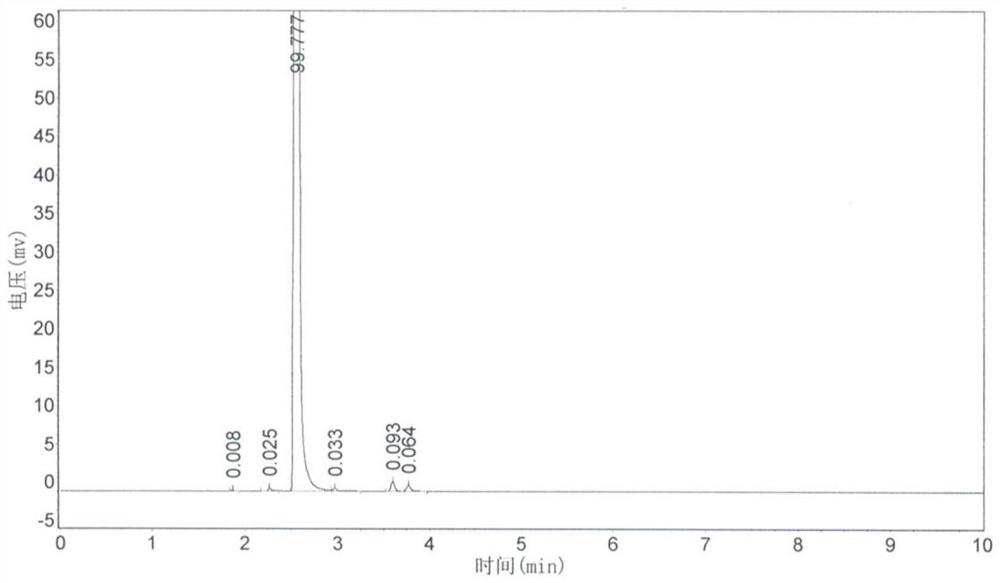
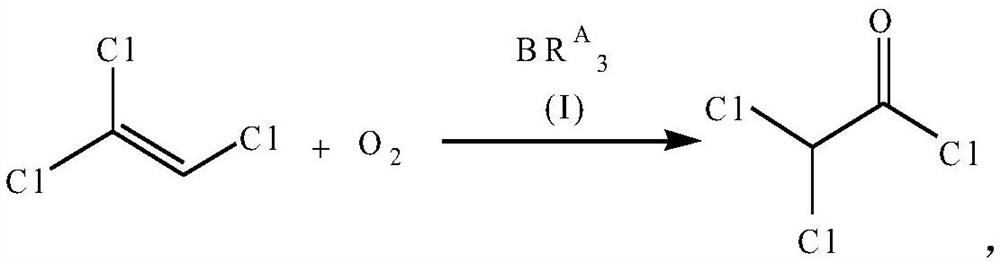
[0043] Step 1. Add 1314g (10mol) of trichlorethylene and 19.6g (0.2mol) of triethylboron in the pressure reactor, then feed dry oxygen, and adjust the pressure of oxygen to 3 atmospheres, then heat up Reaction at 80°C for 10h. After the reaction was completed, atmospheric distillation was carried out, and fractions were collected at a temperature range of 105° C. to 108° C. to obtain 1316 g of a colorless and transparent liquid product, dichloroacetyl chloride, with a yield of 89%. The product was analyzed by gas chromatography and was consistent with the standard sample. The reaction formula is as follows:
[0044]
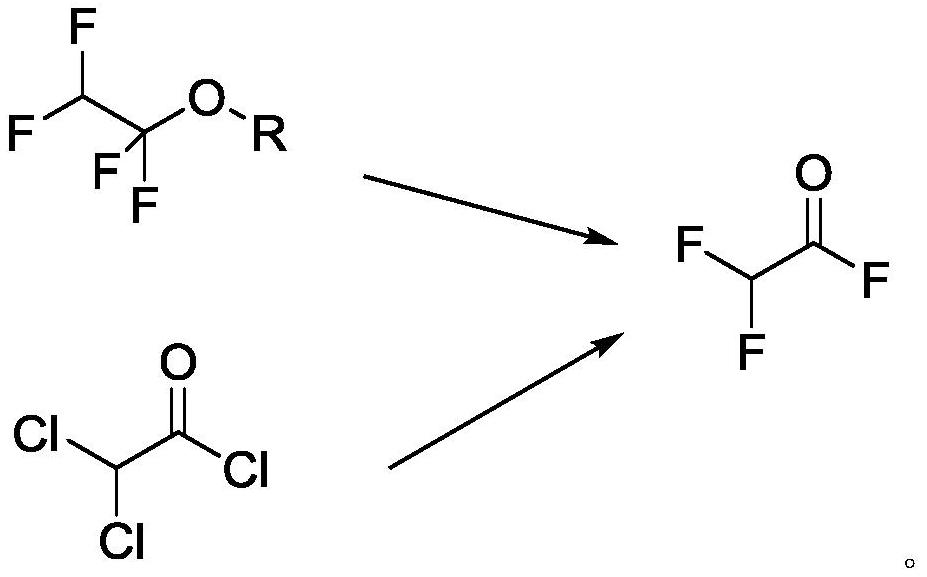
[0045] Step 2. Add 147g (1mol) of dichloroacetyl chloride, 130g of sodium fluoride and 7.4g (0.01mol) of chromium complex catalyst into the pressure reactor, connect the valve and gas conduit, and heat up to 80°C. Open the valve after reacting for 2 ...
Embodiment 2
[0059] The concrete steps of the synthetic method of the difluoroacetyl fluoride of present embodiment are:
[0060] Step 1. Add 1314g (10mol) of trichlorethylene and 18.2g (0.1mol) of tributylboron into the pressure-resistant reactor, then feed dry oxygen, and adjust the pressure of oxygen to 3 atmospheres, then raise the temperature Reaction at 80°C for 11h. After the reaction was completed, atmospheric distillation was carried out, and fractions were collected at a temperature range of 105° C. to 108° C. to obtain 1325 g of a colorless and transparent liquid product, dichloroacetyl chloride, with a yield of 90%. The product was analyzed by gas chromatography and was consistent with the standard sample. The reaction formula is as follows:
[0061]
[0062] Step 2: Add 147g (1mol) of dichloroacetyl chloride, 180g of potassium fluoride and 6.9g (0.01mol) of chromium complex catalyst into the pressure-resistant reactor, connect the valve and gas conduit, and heat up to 80°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com