Preparation method of 3, 4, 5-trifluorobromobenzene
A technology for trifluorobromobenzene and trifluoroaniline, which is applied in the field of preparation of 3,4,5-trifluorobromobenzene, can solve problems such as trichloronitrobenzene that cannot be effectively utilized, and achieves high-value utilization and improved The effect of utilization and process efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
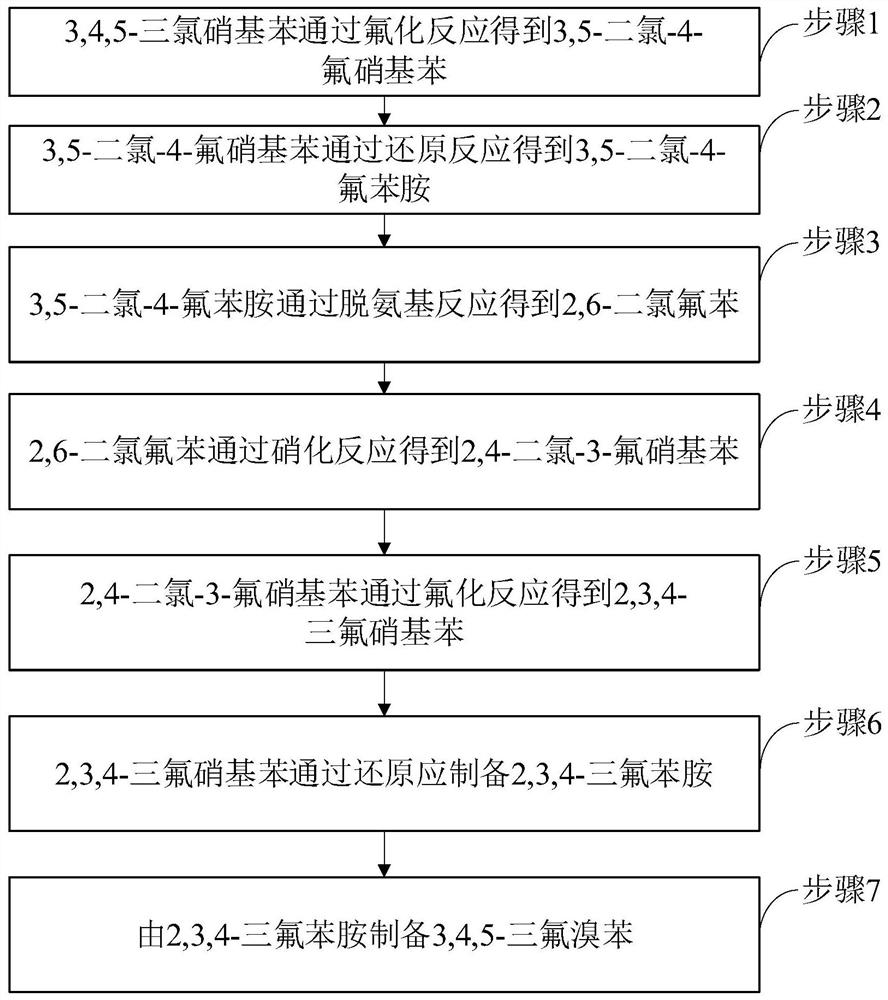
[0045] Such as figure 1 Shown, preparation method comprises the following steps:
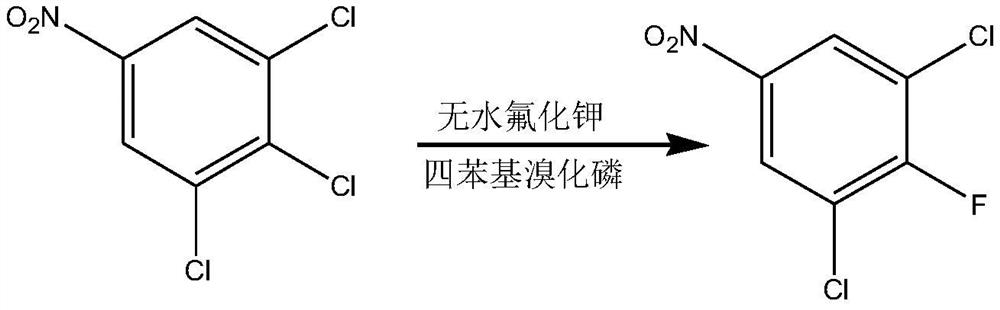
[0046] Step 1, 3,4,5-trichloronitrobenzene is fluorinated to obtain 3,5-dichloro-4-fluoronitrobenzene.
[0047] Specifically, 3,4,5-trichloronitrobenzene and anhydrous potassium fluoride, the molar ratio is 1:(1-1.2), distilled and dehydrated under reduced pressure at 140-150°C for 2-5 hours with stirring. Since 3,4,5-trichloronitrobenzene is a by-product, it contains more water, and the water molecule contains hydroxyl groups, which is not conducive to the fluorination reaction, so it needs to be dehydrated before the reaction. Add an appropriate amount of phase transfer catalyst, raise the temperature to 175-180°C and react for 8-10 hours. Phase transfer catalysts include: tetraphenylphosphine bromide, tetrabutylphosphine bromide, tetrabutylammonium bromide, benzyltriethylammonium chloride.
[0048]It should be noted that the 3,4,5-trichloronitrobenzene in step 1 can directly use the by-pro...
Embodiment 1
[0090] Embodiment 1 (step 1)
[0091] In the flask, 679.5 grams of 3,4,5-trichloronitrobenzene and 191.5 grams of anhydrous potassium fluoride were distilled and dehydrated under reduced pressure at 140°C for 3 hours while stirring, and a phase transfer catalyst (benzyltriethylammonium chloride) was added , heated to 175° C. for 8 hours, cooled, added water to separate layers, and rectified the organic phase under reduced pressure to obtain 504 g of 3,5-dichloro-4-fluoronitrobenzene with a yield of 80%.
Embodiment 2
[0092] Embodiment 2 (step 1)
[0093] In the flask, 679.5 grams of 3,4,5-trichloronitrobenzene and 191.5 grams of anhydrous potassium fluoride were distilled and dehydrated under reduced pressure at 145°C for 2 hours while stirring, and a phase transfer catalyst (tetrabutylammonium bromide) was added, and the temperature was raised React at 178°C for 8 hours, cool, add water to separate layers, and rectify the organic phase under reduced pressure to obtain 491.4 g of 3,5-dichloro-4-fluoronitrobenzene with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com