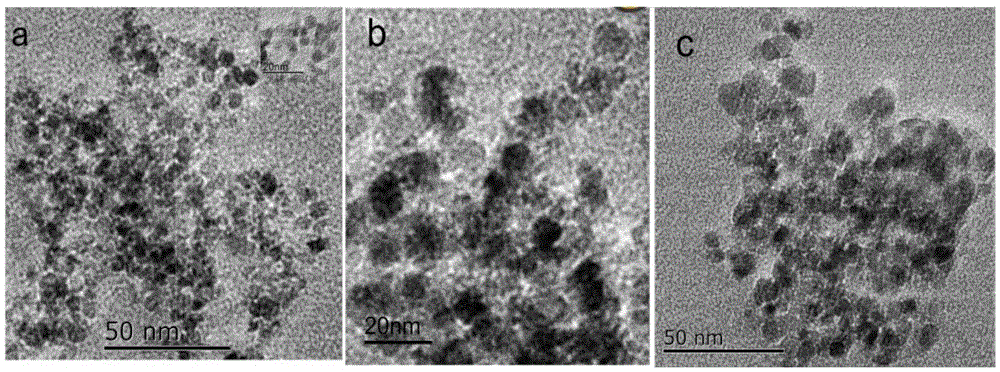
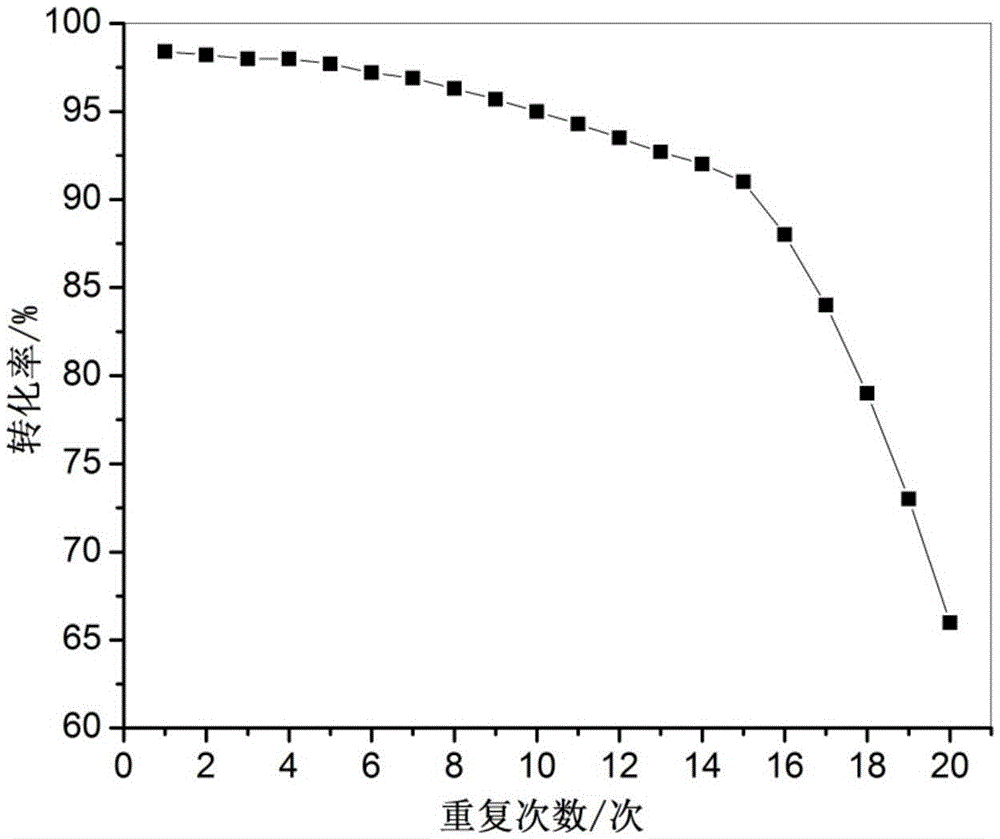
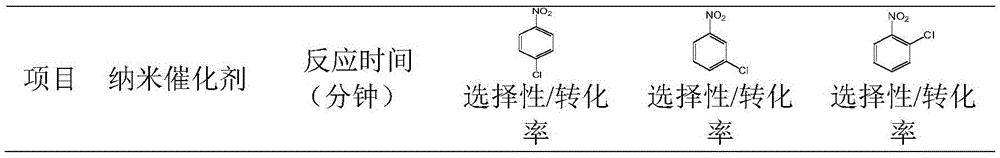
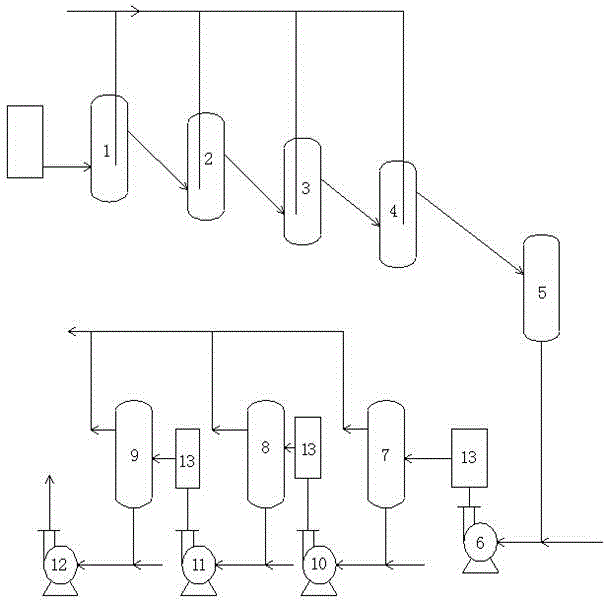
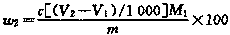Patents
Literature
95 results about "P-chloronitrobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
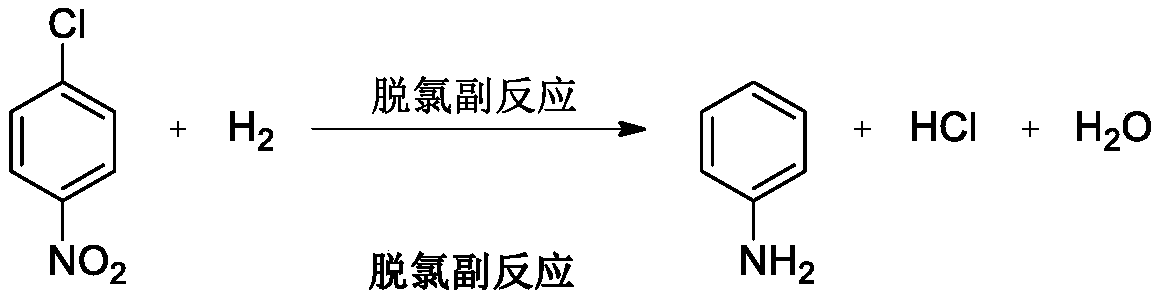
Systemic p-chloronitrobenzene clearance, defined as the ratio of the dose to the area under the plasma concn time curve, decreased with increasing p-chlorobenzene dose. The observations indicated that clearance of p-chloronitrobenzene from the plasma was nonlinear.
Production process of catalytically hydrogenating nitrobenzene halide to synthesize haloarylamine
InactiveCN1506348AInhibition of hydrodehalogenationHigh catalytic activityOrganic compound preparationAmino compound preparationHydrogenation reactionNitrobenzene
The production process is liquid phase catalytic hydrogenation reaction of nitrobenzene halide, such as o-chloronitrobenzene, p-chloronitrobenzene, 3, 4-dichloronitrobenzene and 3-chloro-4-fluoronitrobenzene, etc. with carbon nanotube carried Pd and Pt as hydrogenation catalyst to produce corresponding haloarylamine. The said production process can inhibit hydrodehalogenation, and has the advantages of high selectivity, high yield and high catalyst stability, etc. and thus high practical value.
Owner:ZHEJIANG UNIV OF TECH
Catalyst for synthesizing parachloroaniline by parachloronitrobenzene through hydrogenation and preparation method thereof
InactiveCN101745382AHigh activityHigh selectivityOrganic compound preparationAmino compound preparationActive componentHigh activity
The invention discloses a catalyst for synthesizing parachloroaniline by parachloronitrobenzene through hydrogenation and a preparation method thereof. The active component of the catalyst is Pt, the carrier is attapulgite, and the content of the Pt is 0.1-5wt%. The catalyst not only has high activity, but also effectively inhibits the generation of a dechlorination reaction. Under the condition that the parachloronitrobenzene is completely transformed, the selectivity of 100% for the parachloroaniline is realized.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalyst for preparing halogeno anilin through catalytic hydrogenation of halogeno nitrobenzene and preparation method
InactiveCN101049562AHigh reactivityGood choiceOrganic compound preparationAmino compound preparationActive componentNitrobenzene
A catalyst with high reaction activity, selectivity and stability for preparing halophenylamine by catalytic hydrogenation of halonitrobenzene is composed of carrier (ZrO2, TiO2, SiO3, or MgO) and active component (Au) (0.5-10 Wt %). Its preparing process includes such steps as providing chloroauric acid as precursor, depositing with urea, and ageing, washing, drying, and calcining at 200-350 deg.C.
Owner:TSINGHUA UNIV
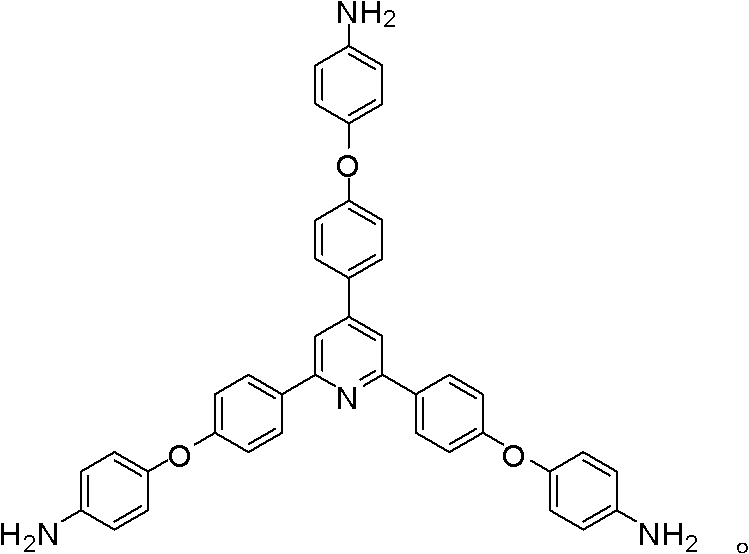
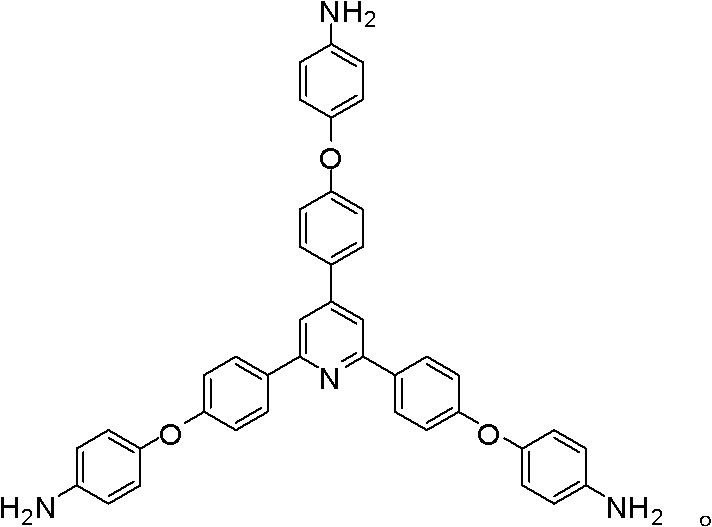
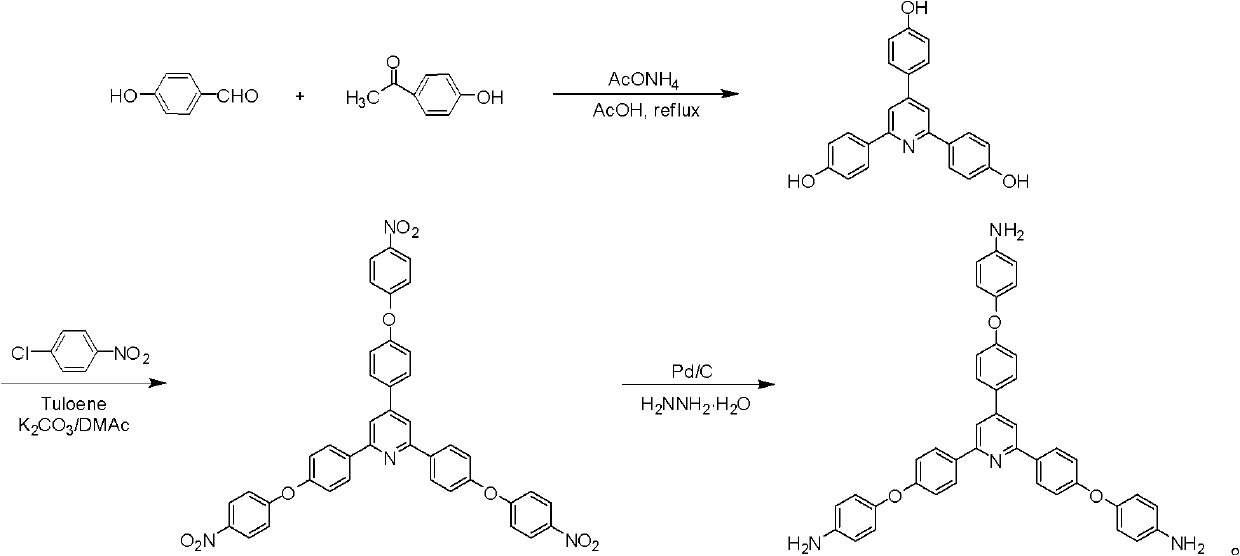
Synthesis of a Triamine Containing a Symmetrical Triarylpyridine Structure and Its Hyperbranched Polyimide
The invention relates to synthesis of a triamine containing a symmetrical triaryl pyridine structure and hyperbranched polyimide thereof. The triamine monomer is BB'2-type aromatic triamine, namely 2,4,6-tris[4-(4-aminophenoxy)-phenyl] pyridine the structure of which is shown in the specification. The triamine is prepared by the following three steps: firstly reacting p-hydroxybenzaldehyde with p-hydroxyacetophenone in acetic acid to obtain 2,4,6-tris(4-hydroxyphenyl) pyridine, then reacting 2,4,6-tris(4-hydroxyphenyl) pyridine with p-chloronitrobenzene to obtain 2,4,6-tris[4-(4-nitrophenoxy)-phenyl] pyridine, and finally reducing the trinitro compound by use of hydrazine hydrate in the presence of a carbon-supported palladium catalyst to synthesize 2,4,6-tris[4-(4-aminophenoxy)-phenyl] pyridine. The hyperbranched polyimide of the triamine is an amino-terminated or anhydride-terminated hyperbranched polyimide polymer obtained by polymerization of the triamine monomer and a commercial dianhydride monomer. The prepared hyperbranched polyimide has excellent high-temperature resistance, solubility and optical properties; and the hyperbranched polyimide polymer material has great application value and prospects in the fields of photosensitive, optical waveguide and gas permeation separation membranes and other materials.
Owner:HUBEI UNIV
Process for synthesizing iron-nickel alloy nano particle catalyst for selective hydrogenation
InactiveCN101352684AOrganic reductionMetal/metal-oxides/metal-hydroxide catalystsSuperparamagnetismSolvent
The invention relates to a method for preparing and separating monodisperse superparamagnetism iron-nickel alloy nano-particle catalyst used for selective hydrogenation. In the method, Ferric acetylacetonate and Nickel acetylacetonate are taken as raw materials, liquid paraffin is taken as reaction medium, trioctyl-phosphine and oleyl amine are taken as stabilizer (also called protecting agent or surface modifier) and polyol is taken as morphology modifier. The product obtained is iron-nickel alloy nano-particle. The method adding magnetic field to assist solvent extraction can separate the iron-nickel alloy nano-particle from the reaction system. The iron-nickel alloy nano-particle can be dispersed into organic solvents again such as chloroform, petroleum ether, and the like, without the generation of precipitation. The nano-particle is used as the catalyst for the selectively hydrogenation of chloro-nitrobenzene, the convention rate of reactants can reach 70 percent and the selectivity thereof is 98 percent.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Production method of highly pure 2,4-dinitrochlorobenzene
InactiveCN104045563AReduce productionEasy to useNitro compound preparationChemical industryNitration
A production method of highly pure 2,4-dinitrochlorobenzene relates to the technical field of the chemical industry. The method comprises the following steps: adding a nitric acid and sulfuric acid mixed acid into a nitration kettle, adding p-chloronitrobenzene in a dropwise manner, heating the nitration kettle to 105DEG C, stopping stirring, allowing the obtained material to stand for layering, adding a waste acid into an extraction pot, adding p-nitrochlorobenzene into the extraction after the extraction in order to react with residual nitric acid in the waste acid, extracting organic matters in the extracted acid, allowing the obtained solution to stand for layering, inputting the waste acid into a concentration workshop section, concentrating, mechanically using, recovering the extracted p-nitrochlorobenzene as a next batch nitration material, adding a qualified nitrated liquid from the nitration kettle into a neutralization water washing pot, adding Na2CO3.10H2O, washing to neutrality, stopping stirring, and allowing the obtained solution to stand for layering in order to obtain a finished product. The method has the advantages of simple and convenient preparation, small waste acid output, less equipment investment, high out degree and convenient operation, and the prepared 2,4-dinitrochlorobenzene has the advantages of good use effect, safety and reliability.
Owner:ANHUI HUARUN PAINTS
Preparation method of 2,4-dichloro fluorobenzene
InactiveCN101134712AEasy to recycleAvoid the stenchPreparation by halogen replacementNitrobenzenePotassium
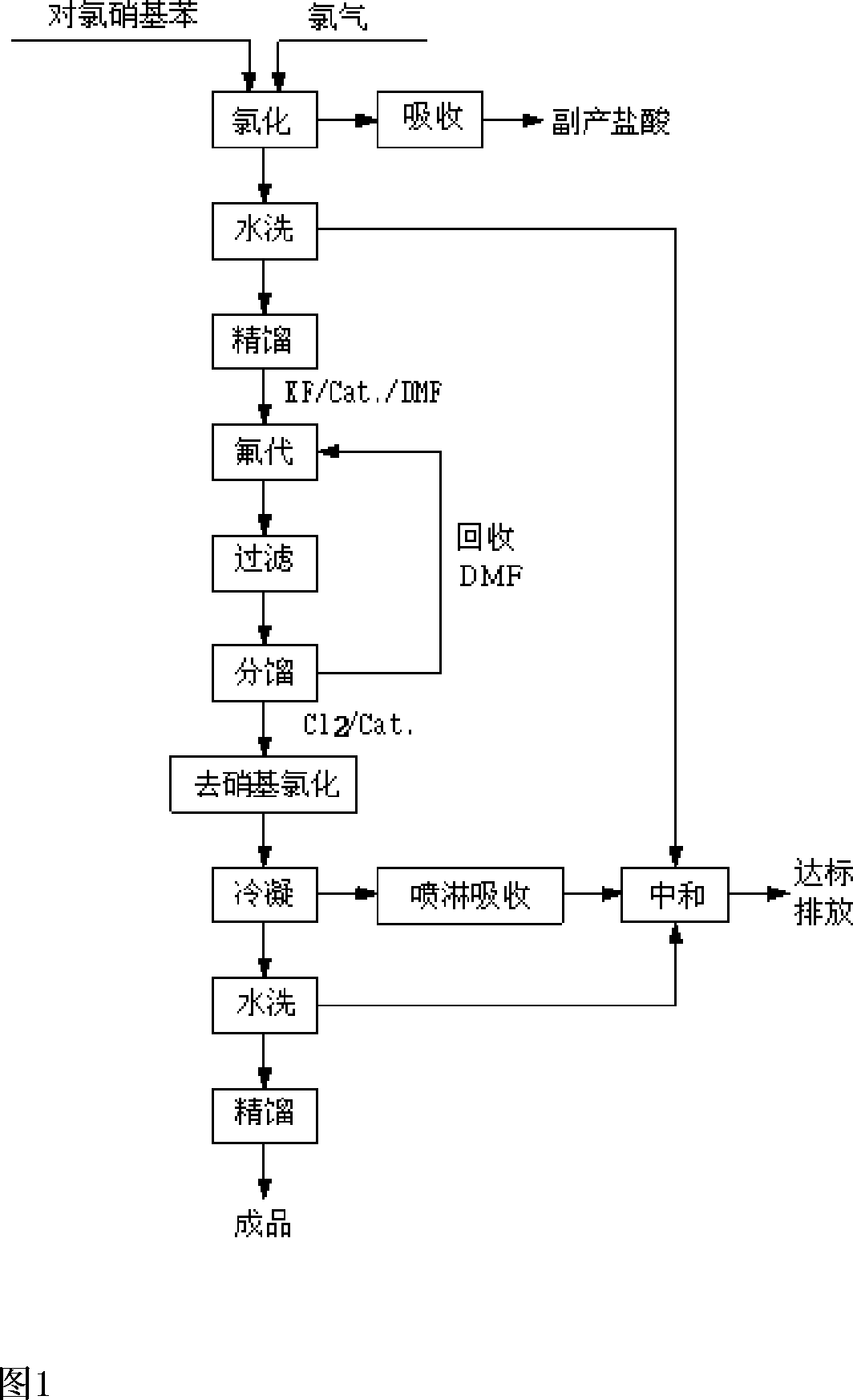
The process of preparing 2, 4-dichlor fluorobenzene includes the following steps: 1. chloridizing parachloro nitrobenzene with chlorine to produce 3, 4-dichloro nitrobenzene; 2. dissolving 3, 4-dichloro nitrobenzene in solvent, and heating to produce fluoro reaction in the presence of catalyst to produce 3-chloro-4-fluoro nitrobenzene and potassium chloride; and 3. reacting 3-chloro-4-fluoro nitrobenzene and chlorine while heating and in the presence of catalyst to eliminate nitro group and produce 2, 4-dichlor fluorobenzene and nitro acyl chloride. The present invention has reasonable reaction path, cheap facile material, low cost, high reaction selectivity, high product quality and high safety.
Owner:浙江省常山长盛化工有限公司
Support catalysts for p-chloronitrobenzene selective hydrogenation and preparation method thereof
InactiveCN101342487AHigh catalytic activityImprove catalytic selectivityOrganic compound preparationAmino compound preparationHydrogenation reactionSolvent
The invention relates to a method to prepare a load catalyst of parachloroaniline used to prepare parachloronitrobenzene hydrogenation and an application method thereof. During preparation of the catalyst, AlO(OH), r-Al<2>O<3>, SiO<2> and SBA-15 are used as carriers respectively to compare the performances of Ru catalyst after loading different carriers. Because the AlO(OH) has excellent dispersing performance in water, Ru nano particles are dispersed evenly, during the reaction for preparing parachloroaniline by using parachloronitrobenzene hydrogenation, excellent catalytic activity is achieved. The temperature and the solvent are changed during the process of catalyst preparation, and ultrasonic assistance is introduced to optimize the method of catalyst preparation. The catalyst has high activity, high selectivity, and has the advantages of that the stability is high, the separation technique is easy and the precious metal is easy to reclaim; the method is applicable to the reaction for preparing parachloroaniline by using parachloronitrobenzene hydrogenation, and excellent hydrogenation activity and selectivity are achieved during the reaction.
Owner:NANKAI UNIV
Catalyst for catalyzing selective hydrogenation of chloro nitro-compound
ActiveCN107999116AHigh catalytic activityImprove conversion rateMolecular sieve catalystsOrganic compound preparationDispersityNitro compound
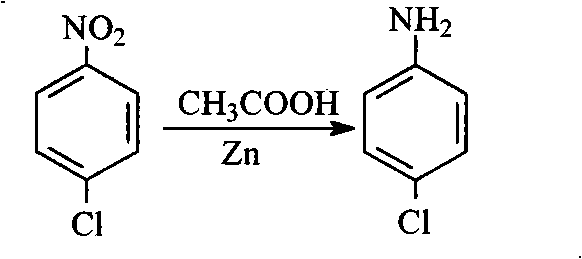
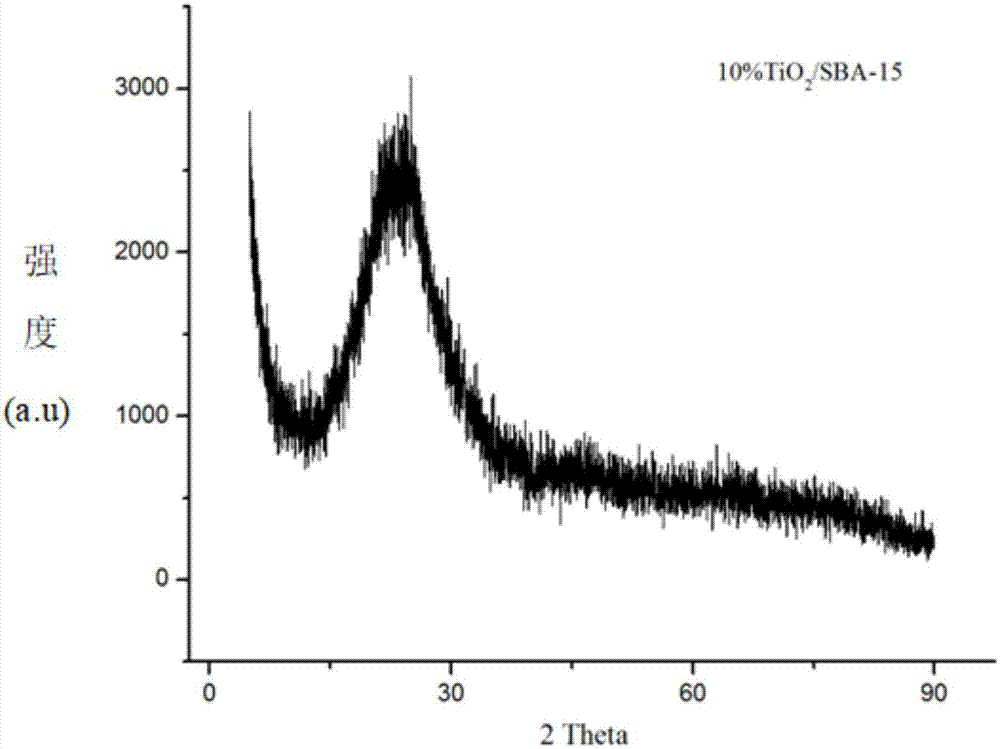
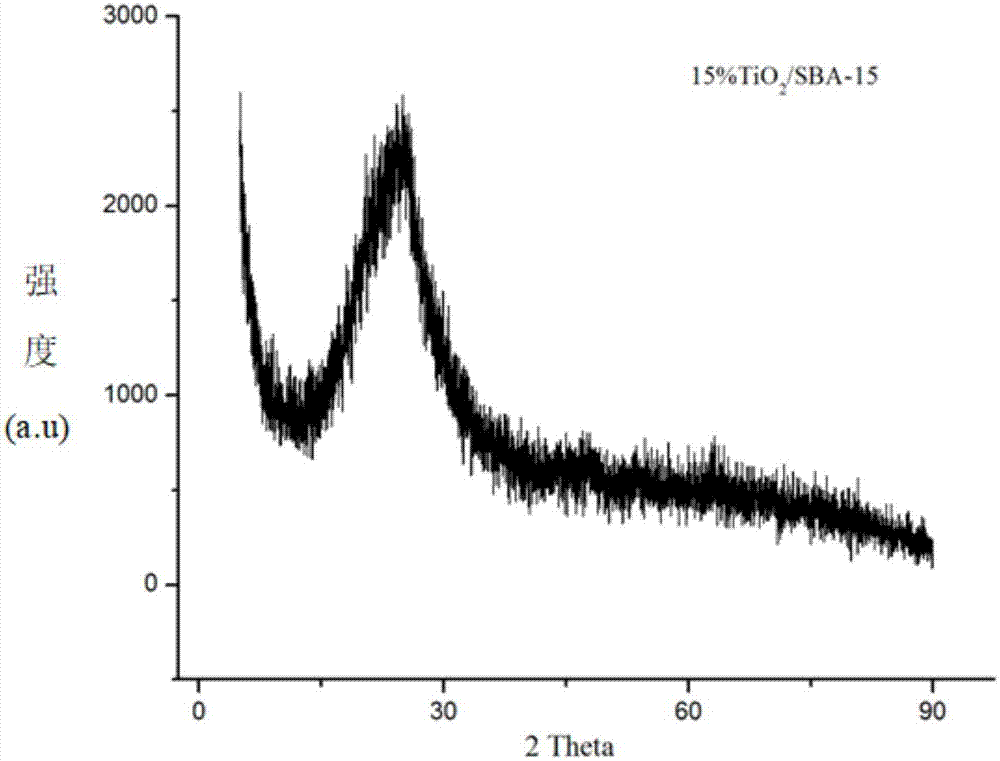
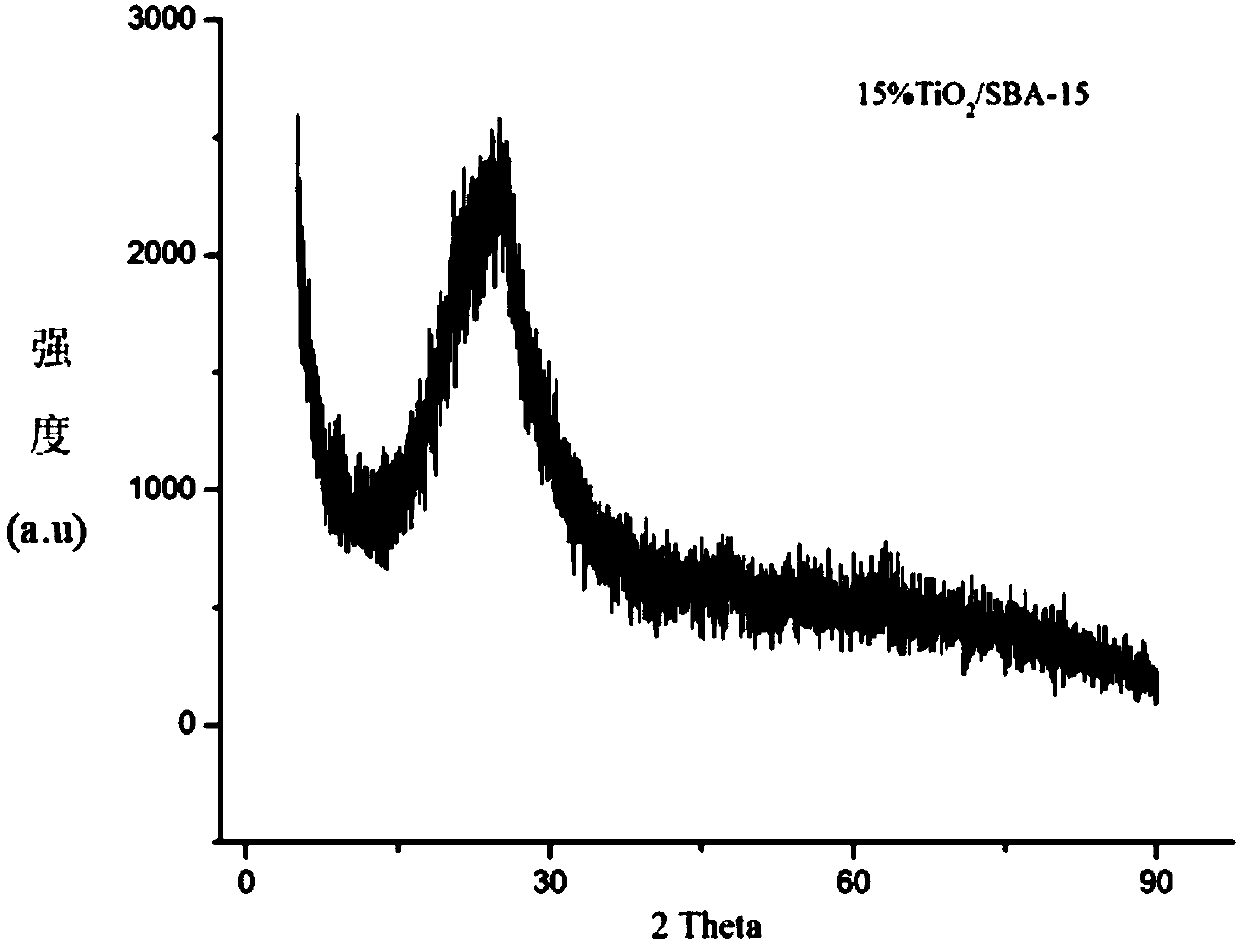
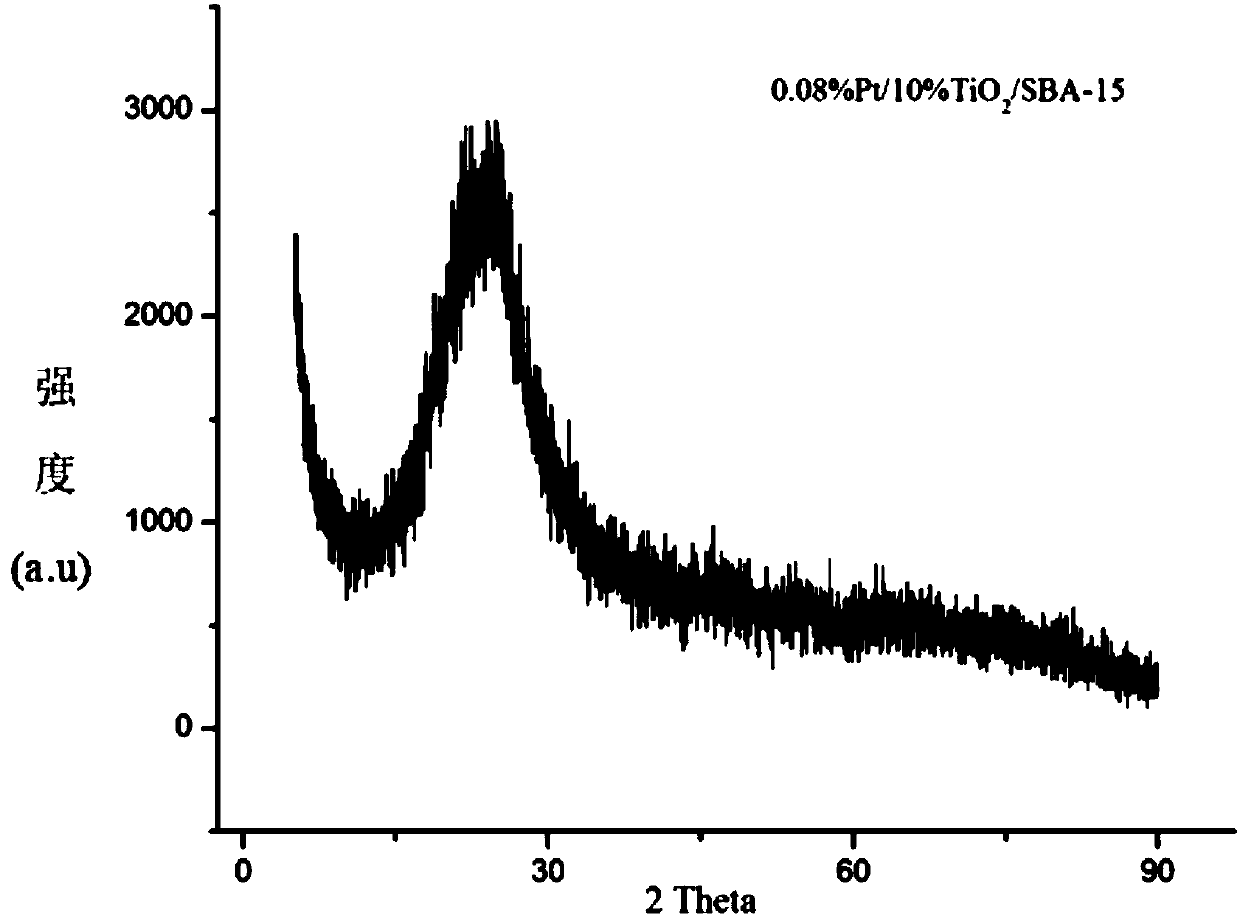
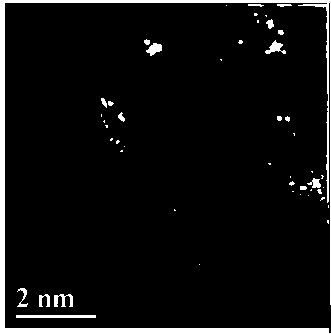
The invention firstly provides a catalyst for catalyzing selective hydrogenation of a chloro nitro-compound. The catalyst is a Pt / TiO2 / SBA-15 catalyst or a Pt / ZrO2 / SBA-15 catalyst, and the content ofplatinum in the catalyst is 0.01-0.3 wt%, preferably 0.05-0.15 wt%, more preferably 0.08-0.10 wt%. The invention further correspondingly provides a preparation method of the catalyst and application of the catalyst in the reaction of selective hydrogenation of parachloronitrobenzene to prepare parachloroaniline. The catalyst shows excellent catalytic activity when used for catalyzing selective hydrogenation of parachloronitrobenzene to prepare parachloroaniline, the conversion rate and the selectivity are very high and both can reach up to 99% or above, and dechlorinating side reactions are greatly reduced. The preparation method of the composite nano-structure catalyst used in the method is simple, the production period is short, the platinum capacity is low, thus, the cost of the catalyst is low, and no reducing agent needs to be added during preparation. The dispersity of platinum is good, and catalytic activity is high.
Owner:XIANGTAN UNIV
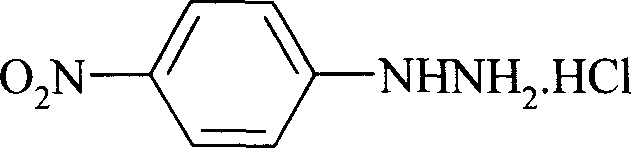
Preparation process of p-nitro phenyl hydrazine hydrochloride
InactiveCN100999483AMild reaction conditionsSimple and safe operationHydrazine preparationHydrazine compoundNitrobenzene
The invention relates to a p-Nitrophenylhydrazine muriate preparation methods. In halogenated hydrocarbonand water two-phase system, take p-cl-nitrobenzene as raw material to take reaction with hydrazine hydrate under catalyst, obtain p-Nitrophenylhydrazine, then through hydrochloride to obtain product of the invention. With this method, the purity of p-Nitrophenylhydrazine muriate can achieve more than 99%, 80 to 85% yield.
Owner:SHANGHAI CHEM REAGENT RES INST
Method for using parachloronitrobenzene to prepare parachloroaniline through selective hydrogenation
ActiveCN108031485AHigh catalytic activityImprove conversion rateMolecular sieve catalystsOrganic compound preparationDispersityPlatinum
The invention provides a method for using parachloronitrobenzene to prepare parachloroaniline through selective hydrogenation. The method comprises the steps that a catalyst containing platinum and titanium dioxide to catalyze parachloronitrobenzene to prepare parachloroaniline through selective hydrogenation under a hydrogen environment and a heating condition, and a dechlorinating side reactionsin the hydrogenation process are sharply decreased, wherein the catalyst is Pt / TiO2 / SBA-15 catalyst. The catalyst is used for presenting superior catalytic activity in the process of catalyzing parachloronitrobenzene to prepare parachloroaniline through selective hydrogenation, the conversion rate and the selectivity are very high, both can reach 99% or more, and the dechlorinating side reactionsare sharply decreased. The composite nanostructure catalyst Pt / TiO2 / SBA-15 used in the method is simple to prepare, short in production cycle, and low in platinum loading capacity, accordingly the catalyst cost is low, high temperature treatment is not needed, and a reduction agent does not need to be added in the preparation process. The platinum dispersity is good, and the catalytic activity ishigh.
Owner:XIANGTAN UNIV
Method using a carbon onion-supported Pt single atom catalyst for catalytic hydrogenation of aromatic nitro compound
InactiveCN108409579AThe preparation process steps are simple and controllableShort preparation timeOrganic compound preparationChemical recyclingNitro compoundNitrobenzene
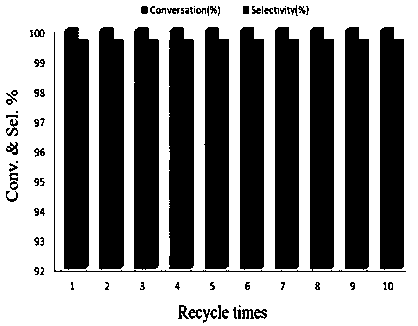
The invention discloses a method using a carbon onion-supported Pt single atom catalyst for catalytic hydrogenation of an aromatic nitro compound, a positive electrode graphite rod electrode is evaporated by arc discharge to provide a carbon source for carbon onion growth, and at the same time, Pt single atoms are formed by reduction of metal cations in a metal salt solution, and embedded in layers of carbon onion. A catalyst sample is washed, dried and used in the selective hydrogenation of the aromatic nitro compound. Results show that the Pt single atom catalyst has the best catalytic performance in ethanol, and the selectivity and the conversion are both greater than 99%. The Pt single atom catalyst prepared by the invention has good stability, not only exhibits excellent catalytic performance in different selective hydrogenation processes, but also can highly-selectively convert halogenated nitrobenzene to halogenated aniline without dehalogenation. The Pt single atom catalyst canbe recycled more than 10 times in the catalytic hydrogenation reaction of p-chloronitrobenzene, and the selectivity and the conversion are both greater than 99%.
Owner:TAIYUAN UNIV OF TECH
Preparation method of 2,2-di[4-(4-amidophenoxy)phenyl]hexafluoropropane
InactiveCN1907952AOrganic compound preparationAmino-hyroxy compound preparationN dimethylformamideBisphenol AF
A preparation method for 2,2-bis-[4-(4-amino-phenoxy)phenyl] hexafluoropropane includes the preparation of 2,2-bis-[4-(4-nitrophenoxy)phenyl]hexafluoropropane and the preparation of 2,2-bis-[4-(4-aminophenoxy)phenyl]hexafluoropropane. The method comprises dissolving bisphenol AF in N,N-dimethylformamide or N,N- dimethylacetamide and toluene or xylene solvent mixture, adding potassium carbonate, refluxing and dehydrating, adding p-chloronitrobenzene at 110-153DEG C for 4-13 hours, filtering and precipitating to obtain 2,2-bis-[4-(4-nitrophenoxy)phenyl]hexafluoropropane, and carrying out reduction with ferric chloride / activated carbon as the catalyst and hydrazine hydrate in methanol or ethanol to obtain 2,2-bis-[4-(4-amino-phenoxy)phenyl]hexafluoropropane with melting point of 164DEG C, purity of 99.4% and yield of 86-90%.
Owner:SHANGHAI RES INST OF SYNTHETIC RESINS
Method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction
ActiveCN101585771ATake advantage ofAvoid direct separationOrganic chemistryOrganic compound preparationChlorobenzenePotassium fluoride
The invention discloses a method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction, comprising steps of: generating a mixture mainly composed of parachloronitrobenzene, o-chloronitrobenzene and m-chloronitrobenzene in process of parachloronitrobenzene, o-chloronitrobenzene by chlorobenzene nitration method; removing low-boiling-point substances by evaporation; then adding anhydrous potassium fluoride and catalyst to the mixture for fluoro-reaction at 150 DEG. C to 250 DEG. C; after reaction, filtering to remove potassium chloride, after rectifying to acquire parachloronitrobenzene, o-chloronitrobenzene, the residual portion via recrystallization acquires m-chloronitrobenzene; the catalyst is quaternary ammonium salt or calixarene; charge rate of chloronitrobenzene mixture and mixture is 1:0.01 to 1. The method of the invention is simple to operate, in scale and changes waste material into things of value, which realizes zero discharge. The method is characterizedin sustainable development, energy saving, consumption reduction and environmentally friendly property.
Owner:浙江省常山长盛化工有限公司
Biological restoration method for p-chloronitrobenzene compound polluted environment
ActiveCN1803228AExtensive metabolic pathwayEfficient use ofBacteriaContaminated soil reclamationRestoration methodSewage
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
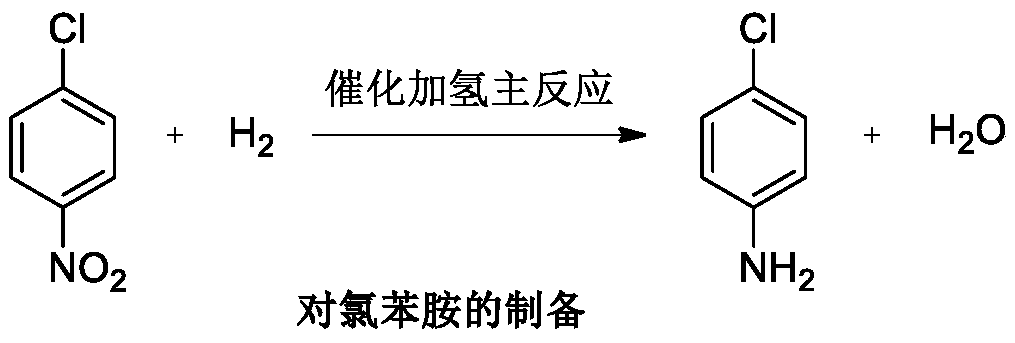
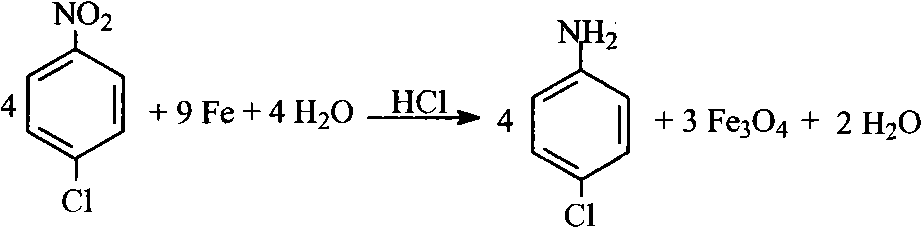
Method for preparing p-chloroaniline through catalytic hydrogenation of p-chloronitrobenzene
InactiveCN102351714AEasy to solveReduce post-processingOrganic compound preparationAmino compound preparationP-chloroanilineOrganic synthesis
The invention belongs to the technical field of organic synthesis and provides a method for performing catalytic hydrogenation on p-chloronitrobenzene with a modified Raney nickel catalyst and preparing p-chloroaniline. The method comprises the following steps: adding p-chloronitrobenzene, the modified Raney nickel catalyst with an additional metal material and a solvent in a high-pressure reaction kettle, wherein the weight ratio of p-chloronitrobenzene to the solvent is 1:2-1:5 and the weight ratio of p-chloronitrobenzene to the catalyst is 1:(0.02-0.06); performing nitrogen replacement, then performing hydrogen replacement, wherein the pressure of hydrogen is 1.0-3.5MPa and the reaction temperature is 60-100 DEG C; and stirring to react for 1-4 hours to obtain the product p-chloroaniline. The method has simple technology, low demand on equipment and low environmental pollution and is easy to realize industrialization; and the reaction conversion rate is 99.9%, the selectivity is not less than 98% and the yield is not less than 95%.
Owner:CHINA PETROCHEMICAL CORP +1
Method for preparing 2, 4-dinitrochlorobenzene by green nitrification technology
InactiveCN107129434AEasy to operateLow priceNitro compound preparationReaction temperatureP-chloronitrobenzene
The invention discloses a method for preparing 2, 4-dinitrochlorobenzene by green nitrification technology. Nitrogen pentoxide / nitric acid serves as a nitrification system, and parachloronitrobenzene serves as a raw material to prepare the 2, 4-dinitrochlorobenzene. When 5 grams of parachloronitrobenzene is used, reaction time is 70 minutes, reaction temperature is 50 DEG C, the concentration of the nitrogen pentoxide is 0.3 gram per milliliter (3 grams of nitrogen pentoxide and 10 milliliters of nitric acid), the yield of the 2, 4-dinitrochlorobenzene is 96.73%, and the purity of the 2, 4-dinitrochlorobenzene is 100%.
Owner:WUHAN UNIV OF SCI & TECH
Process for photo catalytic reduction preparation of p-chloroaniline
InactiveCN1634862AChemically stableLow priceOrganic compound preparationAmino compound preparationP-chloroanilineScavenger
The invention provides a method for reduction preparation of p-chloroaniline by photocatalysis. The process comprises the following steps: solving p-chloronitrobenzene with organic solvent in photocatalysis reactor allowing over 300nm ultraviolet light to pass with the mass ratio of p-chloronitrobenzene to solvent between 1í†40 and 1í†225, adding hole scavenger and photocatalyst with the volume ratio of hole scavenger to organic solvent between 1í†3 and 1í†18 with the concentration of photocatalyst in organic solvent between 0.5g / L and 10g / L, adding nitrogen for removing oxygen, and irradiating for 3 hours to 6 hours by ultraviolet light at normal pressure. The reduction reaction can be carried out at normal temperature without high temperature energy consumption device, initiation light can be ultraviolet light or sunlight, and nano photocatalyst can be titanium dioxide or zinc oxide, etc. The invention has higher reduction conversion and yield.
Owner:TIANJIN UNIV
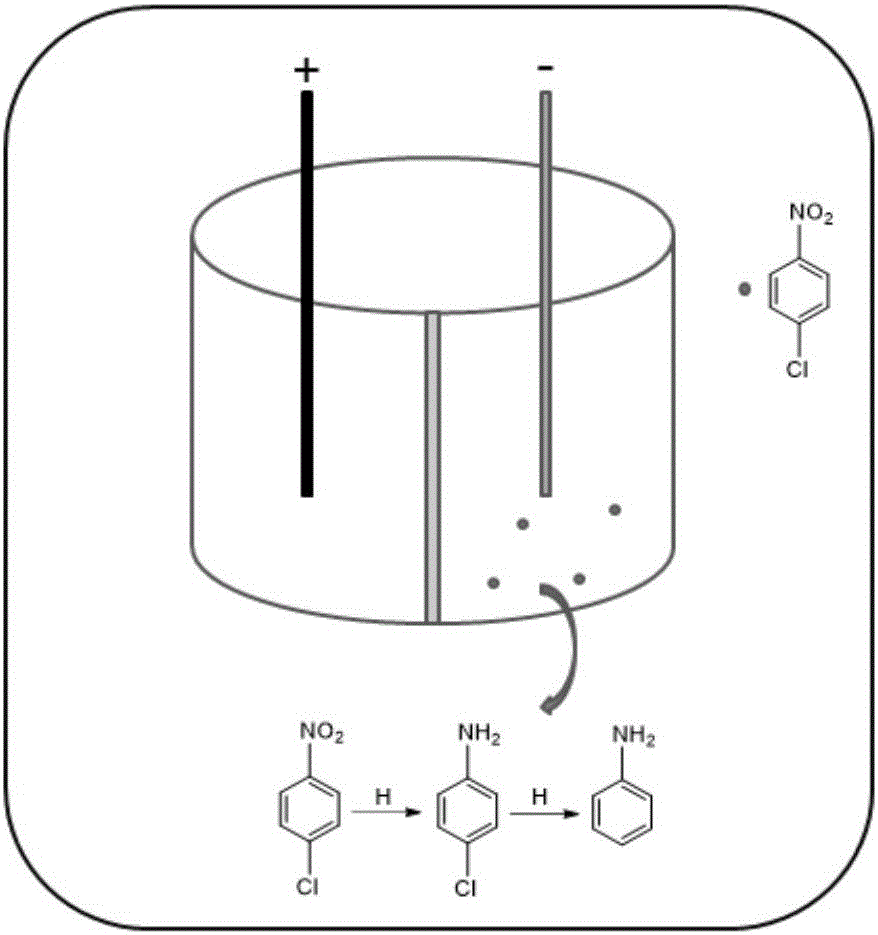
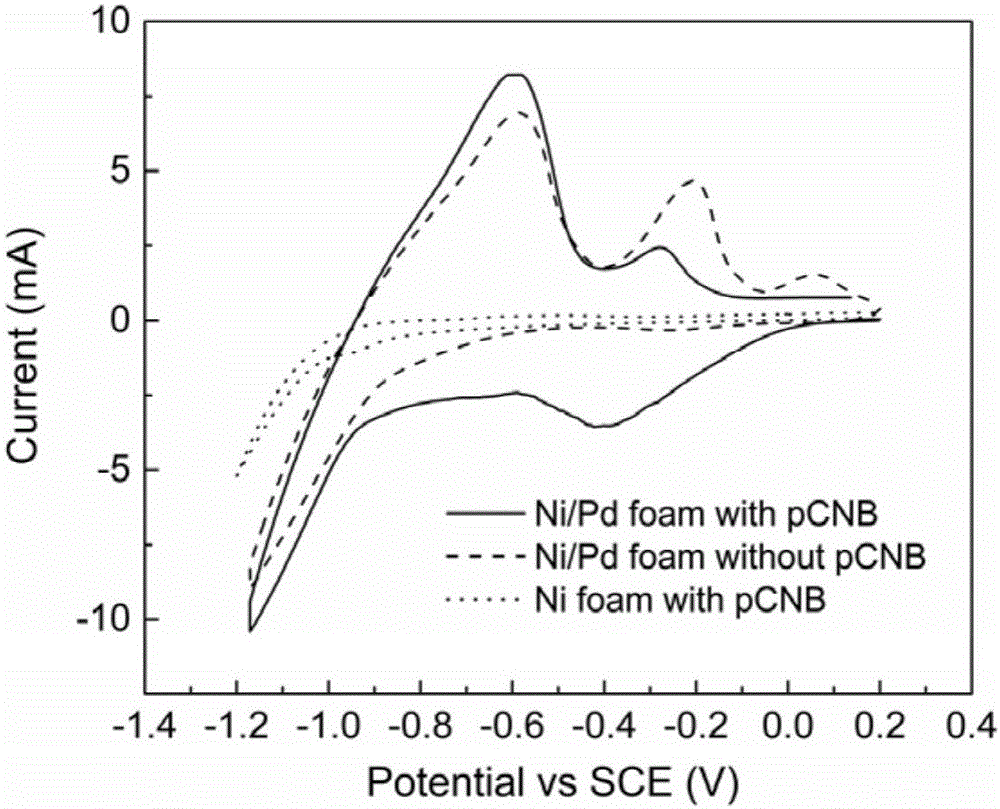
Electrocatalytic dechlorination method for parachloronitrobenzene
InactiveCN105712447AAchieve one-time removalGood dechlorination effectWater contaminantsWater/sewage treatmentChemical treatmentNitrobenzene
The invention discloses an electrocatalytic dechlorination method for parachloronitrobenzene. The method comprises the following steps: loading precious metal palladium on a foam nickel material with a three-dimensional structure by an impregnation method to obtain a cathode of an electrocatalytic reactor; and introducing parachloronitrobenzene-containing wastewater into the electrocatalytic reactor to fill the whole reactor, wherein electrochemical treatment is started under constant current, the parachloronitrobenzene experiences a reduction reaction in a cathode chamber and is reduced into parachloroaniline under the effect of hydrogen radical, and then a dechlorination effect can be realized to form aniline due to the excellent performance of the palladium-containing foam nickel electrode. In the invention, metal reduction is replaced by electrocatalytic reduction, the problems of easy passivation and low mass transfer efficiency of metals are solved, the difficulty in dechlorination of parachloronitrobenzene in the traditional electrode is overcome, and the chlorine group of chloronitrobenzene is effectively removed.
Owner:NANJING UNIV
Method for synthesizing 2,4-dichloroaniline
InactiveCN101362699AHigh selectivityImprove product qualityOrganic compound preparationAmino compound preparationHydroxylamineHydroxylamine Hydrochloride
The invention discloses a method for synthesizing 2, 4-dichloroaniline, which adopts parachloronitrobenzene as raw material and comprises the steps as follows: firstly, nitro-benzene is reduced into the corresponding hydroxylamine in the alcohol-water solution of ammonium chloride; secondly, chloro of benzene ring and reduction of hydroxylamine are carried out simultaneously in the hydrochloric acid solution; and thirdly, the pH value is adjusted until the solids are precipitated, and 2,4-dichloroaniline can be obtained after recrystallization by using the alcohol-water solution. The hydrochloric acid in this invention is adopted not only as a source of hydrogen for reduction reaction, but also as a chlorination agent for chlorination reaction, the reduction reaction and the chlorination reaction can be finished at the same time, the invention has the advantages of short process route, no high requirement for devices, simple operation, and high yield of 2,4-dichloroaniline, no usage of expensive catalysts, low raw material and production cost and being suitable for industrial production.
Owner:ZHEJIANG UNIV
Preparation method of 2-amino-5- chlorobenzophenone
InactiveCN107698453AHigh yieldEmission reductionOrganic chemistryOrganic compound preparationPalladium on carbonAmmonium formate
The invention discloses a preparation method of 2-amino-5- chlorobenzophenone. The preparation method comprises the following steps: (1) mixing ethanol with the mass concentration of 95%, sodium hydroxide, parachloronitrobenzene and benzyl cyanide, mixing the mixture and performing ultrasonic oscillation on the mixture at the temperature of 25-35 DEG C for 1h; (2) performing microwave heating on aproduct obtained in step (1) for 10min, adding water in a system, filtering, washing filter residues with methanol three times and drying the filter residues, so as to obtain 5-chlorine-3-phenyl-2, 1-benzisoxazole; (3) mixing methanol, the 5-chlorine-3-phenyl-2, 1-benzisoxazole, a palladium carbon catalyst and ammonium formate, heating and refluxing for 2h, cooling to room temperature, filteringand performing vacuum drying on the filter residues. The method provided by the invention is high in product yield, short in reaction time and less in waste discharge and is environmentally friendly.
Owner:SHAANXI JUJIEHAN CHEM CO LTD
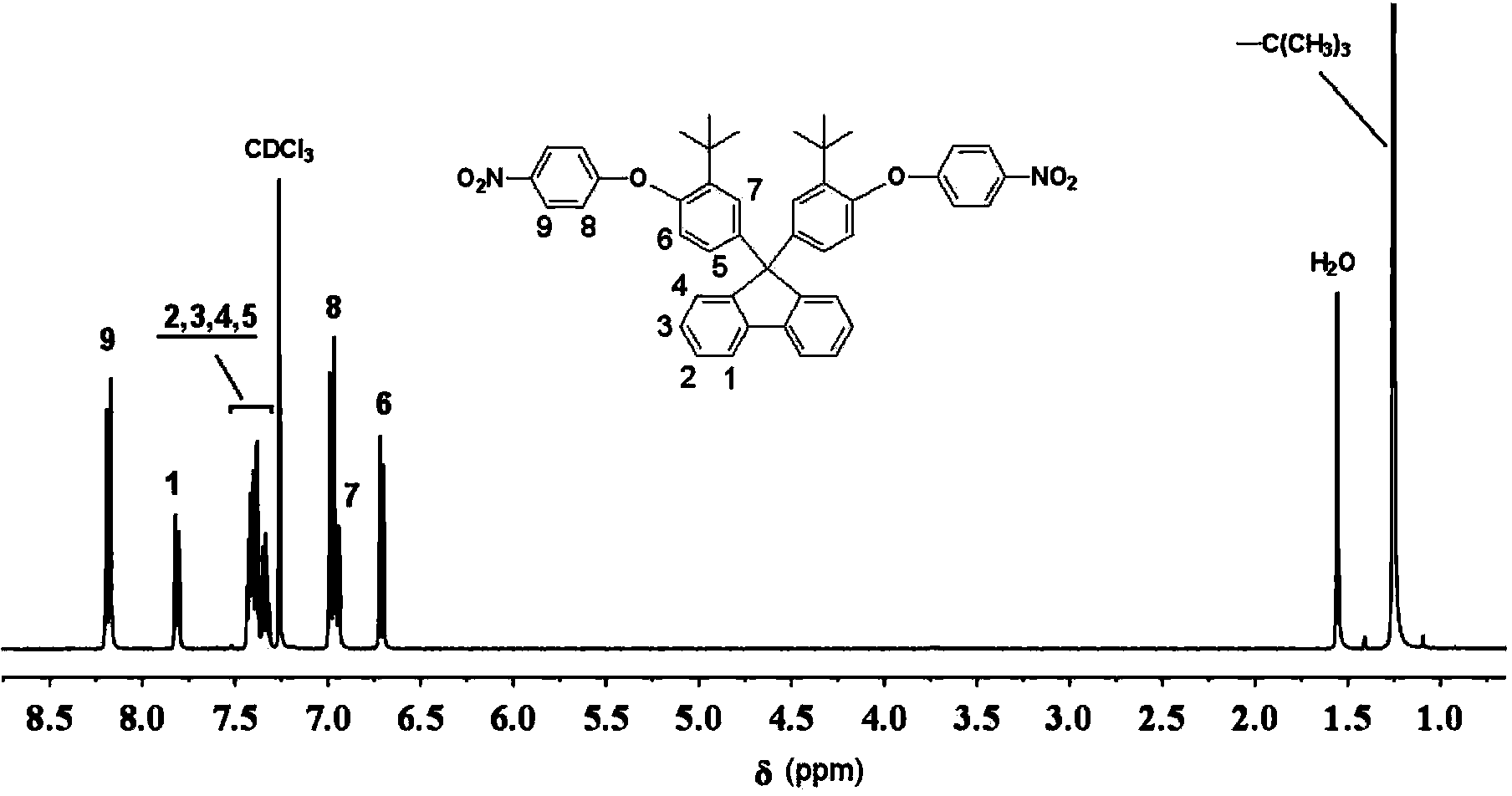
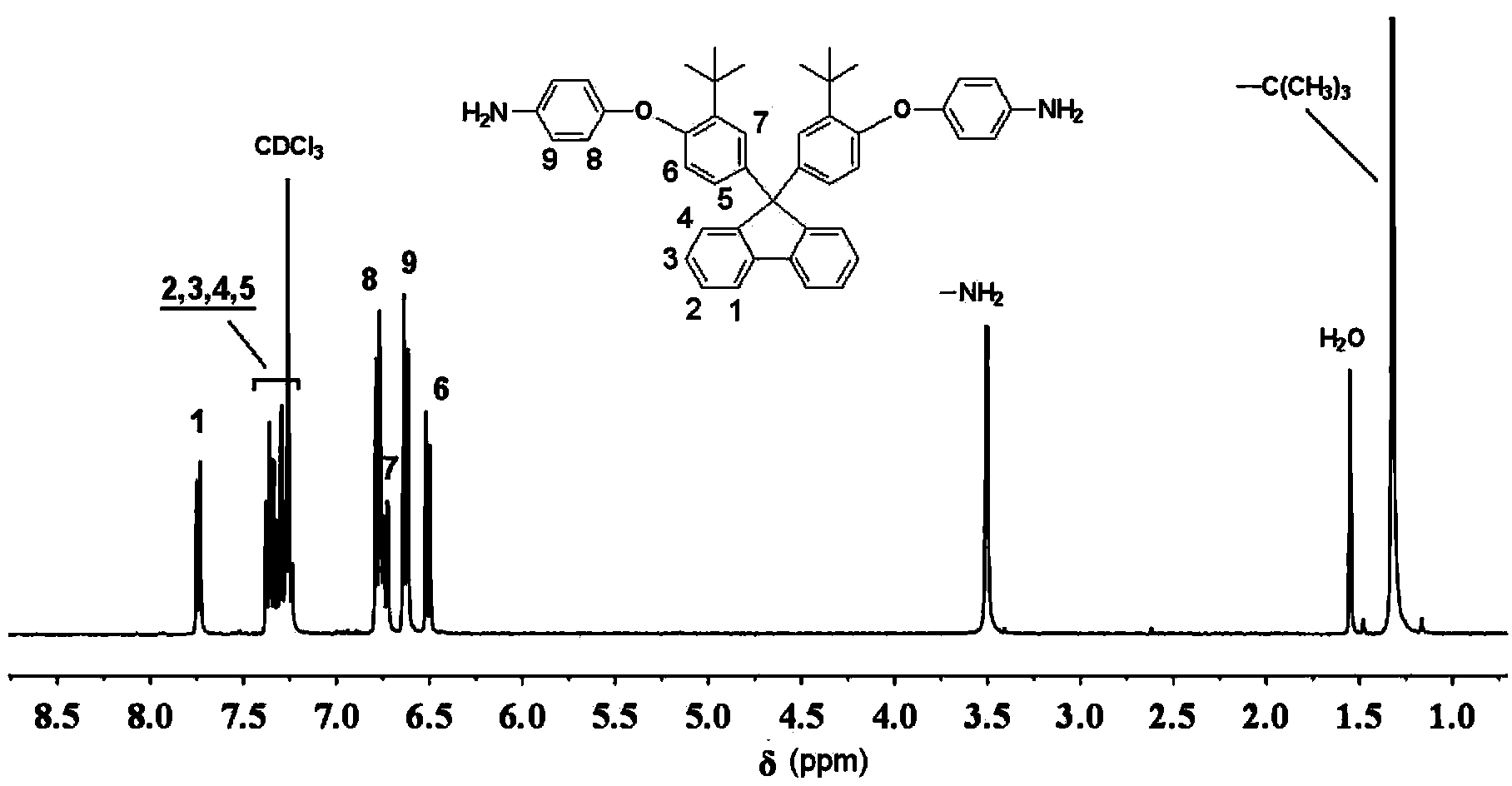
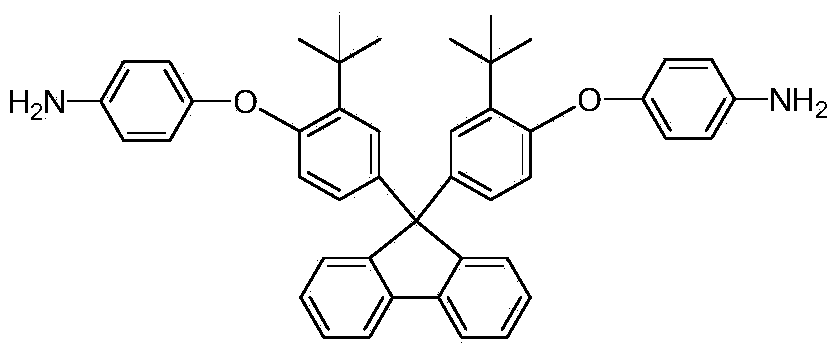
Aromatic diamine monomer containing di-tert-butyl structure and preparation method and application of aromatic diamine monomer
ActiveCN104356011AEasy to separateThe synthesis method is simpleOrganic compound preparationAmino-hyroxy compound preparationNitro compoundPolyetherimide
The invention provides an aromatic diamine monomer containing a di-tert-butyl structure. A preparation method of the aromatic diamine monomer comprises the following steps: a bisphenol compound 9,9-di(3-tert-butyl-4-hydroxyphenyl)fluorine and parachloronitrobenzene react under an alkaline condition to obtain a dinitro compound 9,9-di(3-tert-butyl-4-(4-nitrophenoxy)phenyl)fluorine; then the dinitro compound is subjected to catalytic reduction in an organic solvent to obtain the aromatic diamine monomer 9,9-di(3-tert-butyl-4-(4-amidophenoxy)phenyl)fluorine. The aromatic diamine monomer can be applied to preparation of soluble polyetherimide, and the prepared polyetherimide has a favourable film-forming property, a high thermal property and an excellent gas separation property.
Owner:CHANGZHOU UNIV
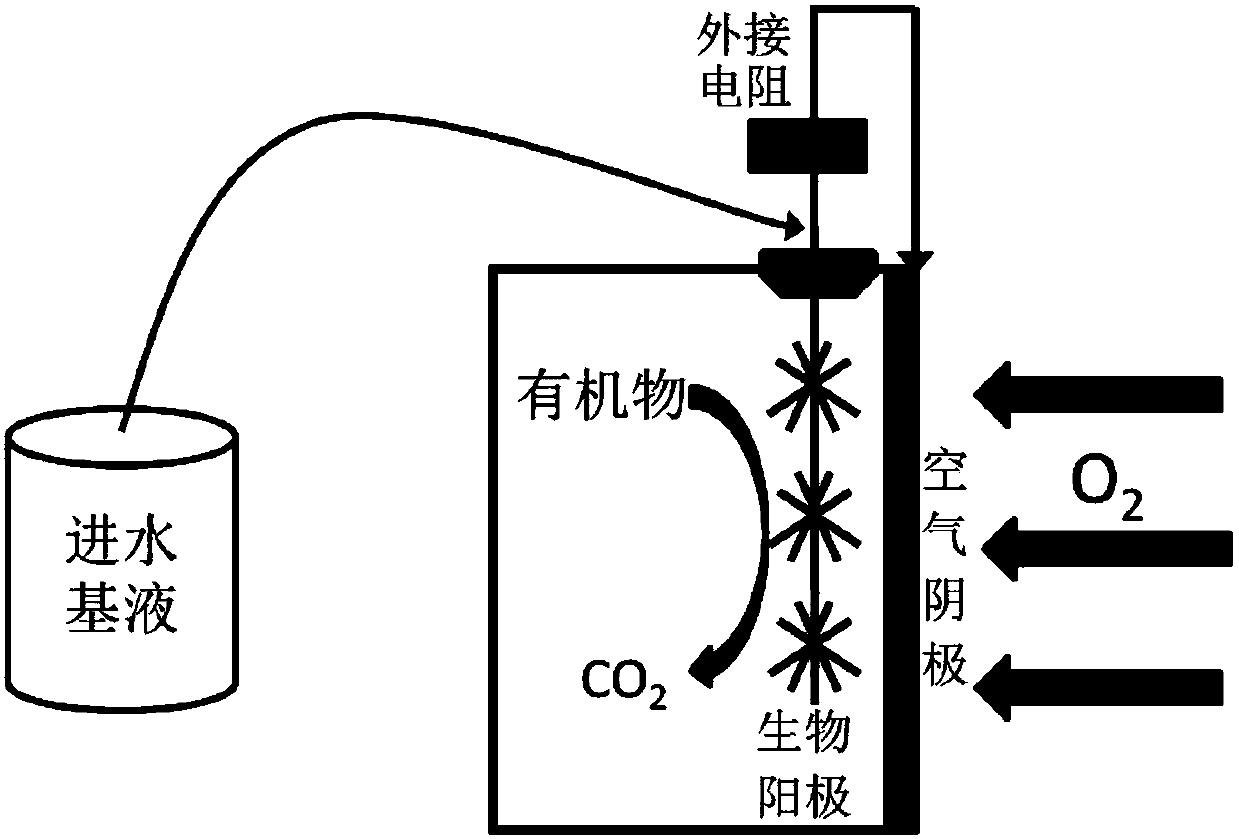
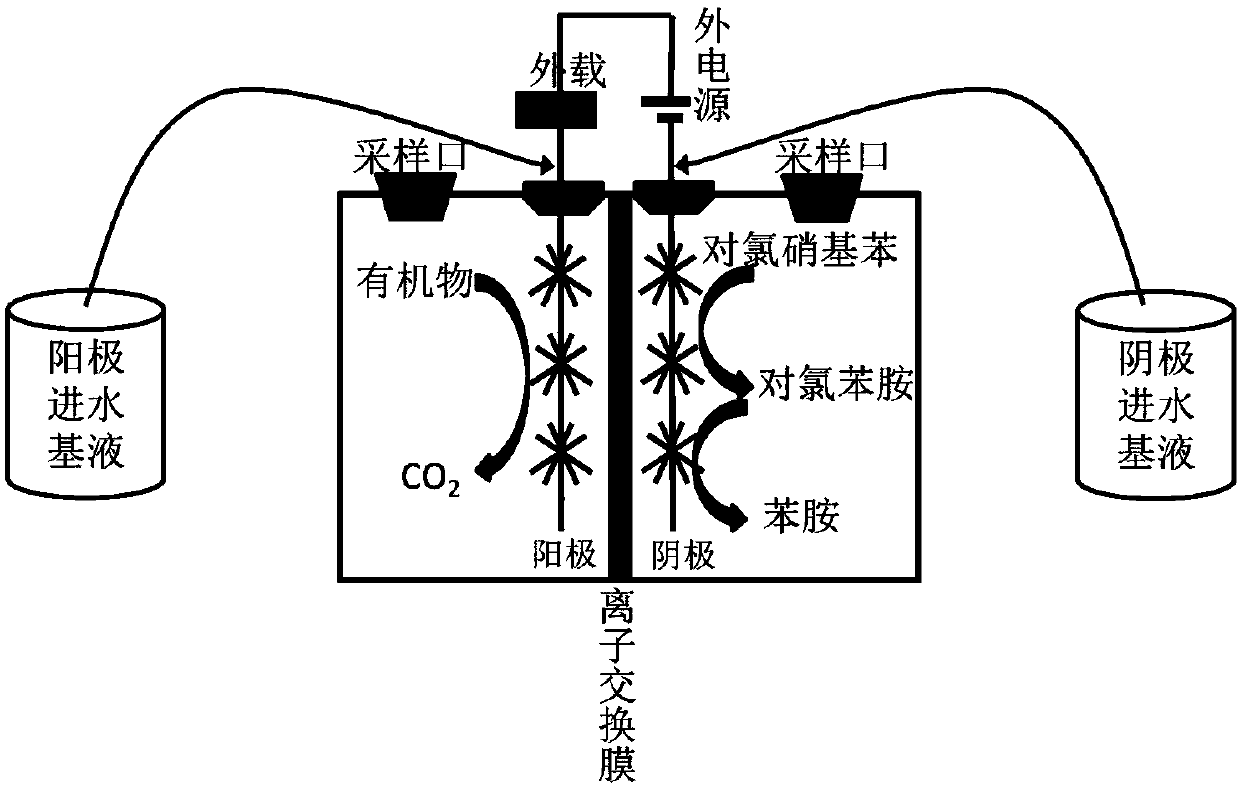
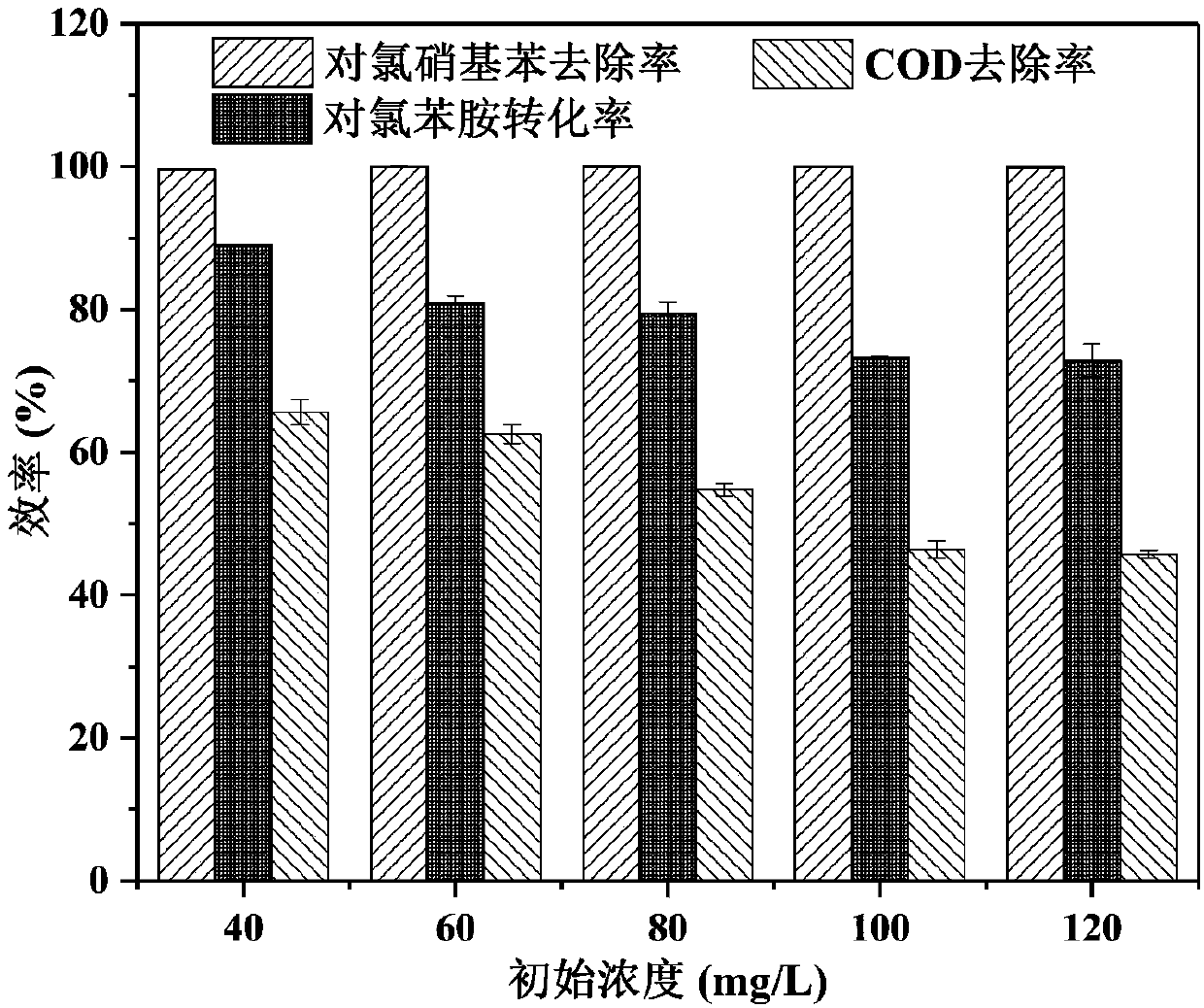
Method for high-efficiency degradation of p-chloronitrobenzene through microbial electrocatalysis
InactiveCN107673463AHigh activityReduce rateTreatment by combined electrochemical biological processesWater contaminantsSludgeSpecific function
The invention discloses a method for high-efficiency degradation of p-chloronitrobenzene through microbial electrocatalysis. The method comprises the following steps: carrying out single-chamber longitudinal domestication on electrochemically active microorganisms; placing inoculated sludge and a culture medium in an anaerobic bottle, and carrying out gradient domestication and enrichment on p-chloronitrobenzene degrading bacteria; reconstructing a single chamber to form double chambers; adding an anodic water feeding base fluid into an anode chamber, taking out a domestication fluid from theanaerobic bottle, adding the domestication fluid into a cathode chamber, then adding a medium fluid, connecting an external resistor and an external power source to form a closed loop, thereby completing starting of a double-chamber microbial electrocatalysis apparatus, wherein anodes are allowed to enrich the electrochemically active microorganisms and cathodes are allowed to enrich p-chloronitrobenzene bacteria with specific functions; and oxidizing carbon sources in the anode chamber and the cathode chamber under the action of electrochemically active bacteria so as to release electrons, wherein electrons in the anode chamber reach the cathodes via an external circuit and are transferred to p-chloronitrobenzene, and electrons released through oxidation in the cathode chamber are also transferred to p-chloronitrobenzene. The method provided by the invention realizes high-efficiency degradation of p-chloronitrobenzene.
Owner:TIANJIN SEA WATER DESALINATION & COMPLEX UTILIZATION INST STATE OCEANOGRAPHI
Parachloronitrobenzene hydrogenation reduction catalyst capable of being magnetically recycled and preparation method
InactiveCN105413693AImprove catalytic performanceEasy to recycleOrganic compound preparationChemical recyclingCobalt saltWater soluble
The invention relates to a parachloronitrobenzene hydrogenation reduction catalyst capable of being magnetically recycled and a preparation method. Prepared Fe3O4 nanometer magnetic particles and water-soluble cobalt salt are added into water; ultrasonic full mixing is performed under the protection of inert gas, a reducing agent is added, and a target product is obtained after full reaction under ultrasound. Relevant salts such as iron and cobalt which are rich in nature are adopted as a matrix, raw materials are easy to obtain, and the cost is low. The prepared catalyst can be recycled through an external magnetic field due to the fact that the catalyst contains the Fe3O4 nanometer magnetic particles. The grain size of the prepared catalyst is 5-30 nm, so that the specific surface area can be 100-180 m<2> / g, and thus extremely huge promoting effects can be achieved on reagent adsorption and dispersion in the catalytic reaction process, and finally the conversion rate and selectivity reach 100%. The preparation method is simple, low in energy consumption, environmentally friendly and suitable for industrial production.
Owner:SHANDONG NORMAL UNIV
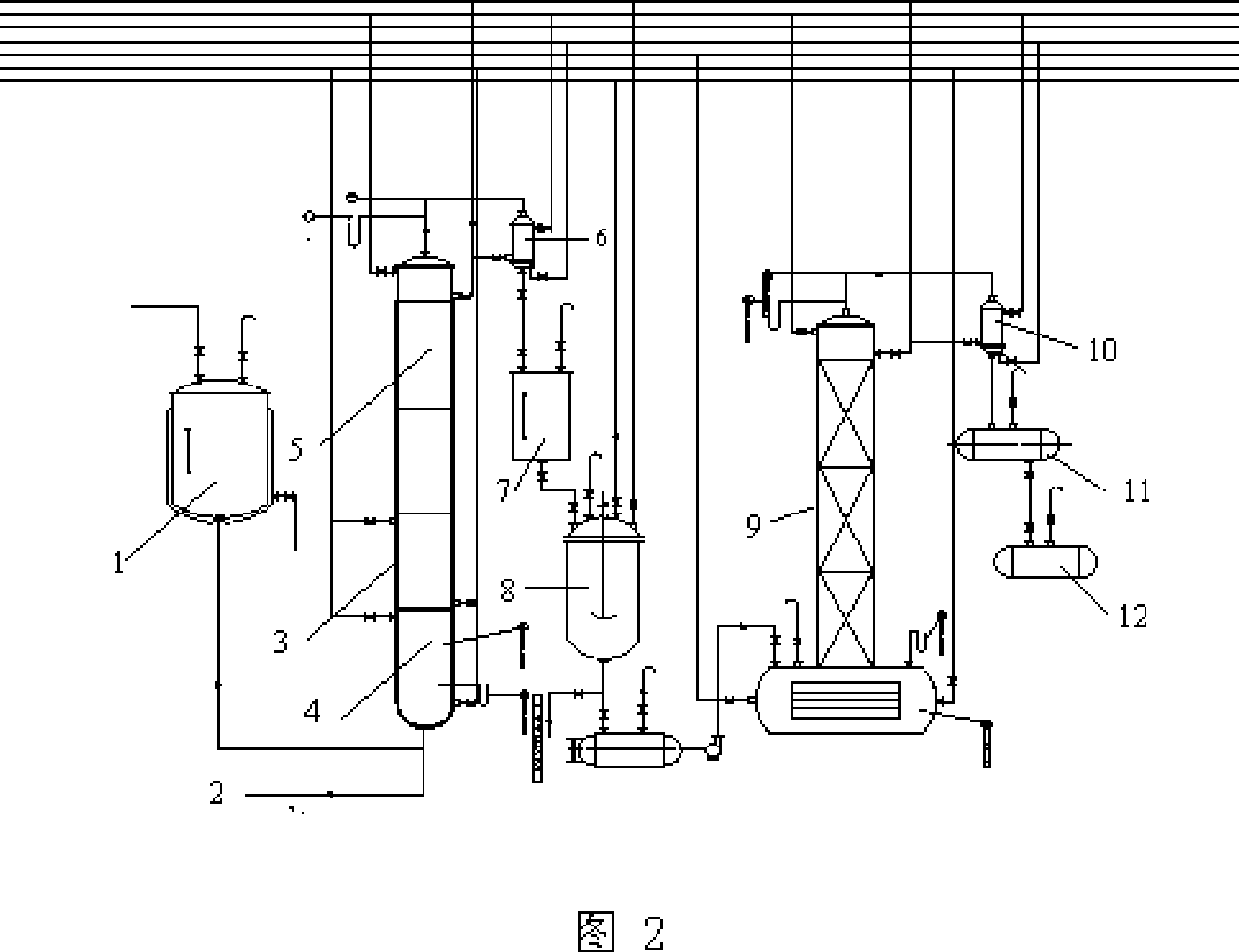
Method and device for producing 3,4-dichloronitrobenzene through continuous kettle type reaction
InactiveCN105418433AEasy to operateHigh level of self-controlOrganic chemistryOrganic compound preparationNitro compoundAutomatic control
The invention relates to the field of chemical production and particularly discloses a method and device for producing 3,4-dichloronitrobenzene through a continuous kettle type reaction. According to the method, parachloronitrobenzene serves as a raw material. The method is characterized in that parachloronitrobenzene and ferric trichloride are prepared into mixed liquor, the mixed liquor enters reaction kettles continuously through a nitro compound pump, and meanwhile chlorine enters the bottom of a chlorination kettle; under the catalyst effect, parachloronitrobenzene and chlorine react to generate 3,4-dichloronitrobenzene; later, by the utilization of the high-order difference among all the reaction kettles, the materials overflow sequentially to enter the reaction kettles connected in series, enter a storage tank finally, and then enter a static mixer through a mixing pump to be mixed with water, multiple washing kettles are connected in series, and qualified materials enter a crude product tank. The process is easy to operate, high in automatic control level, low in production cost, high in stability, safe to operate and suitable for large-scale industrial production, and effectively saves labor, and the purpose of continuously chlorinating and washing 3,4-dichloronitrobenzene is achieved.
Owner:山东沾化天九化工有限公司
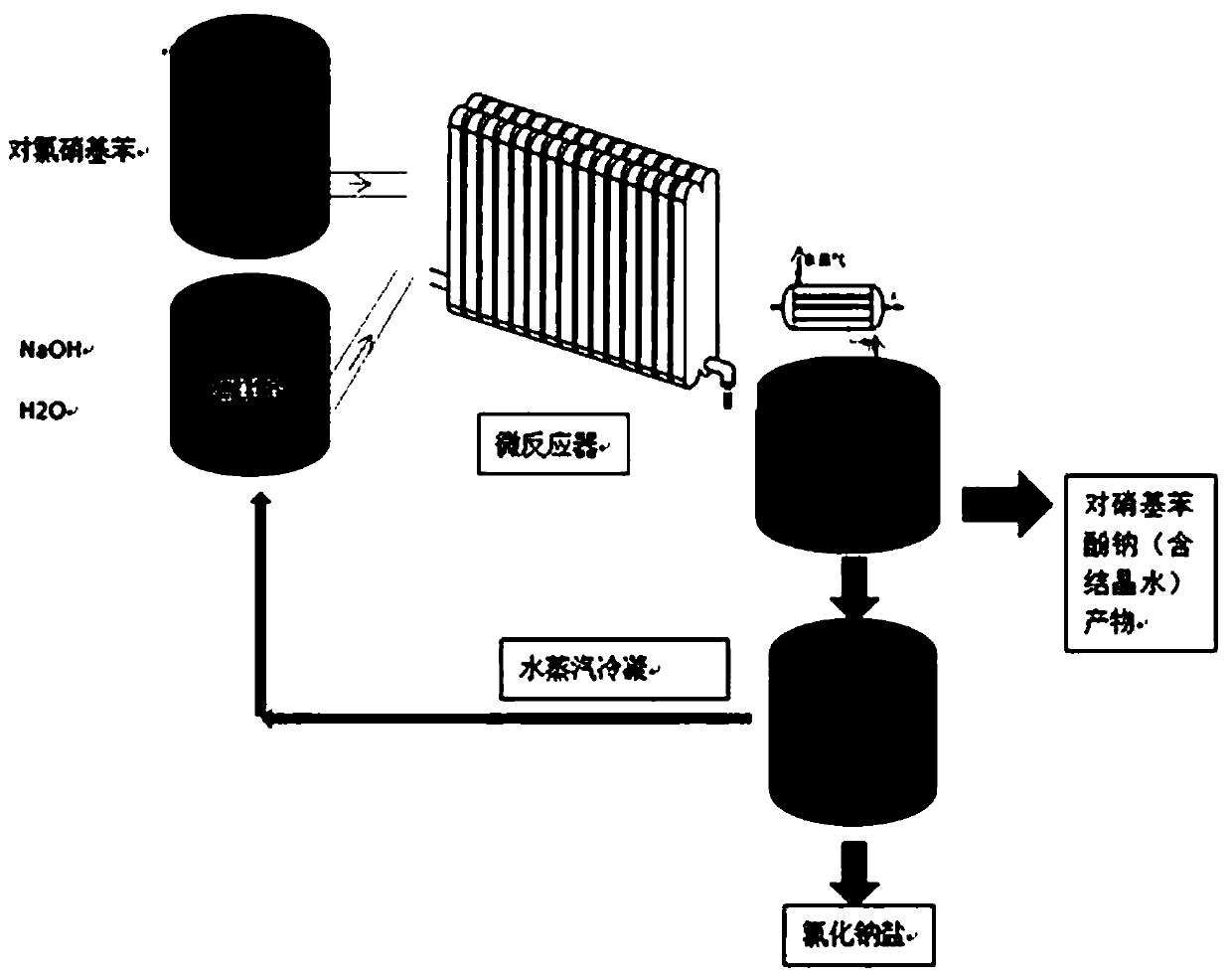
Preparation method of sodium p-nitrophenolate
PendingCN111004126AWell mixedEfficient heat transferOrganic chemistryOrganic compound preparationNitrobenzeneNitrogen gas
The invention discloses a preparation method of sodium p-nitrophenolate. The preparation method comprises the following steps: a) heating and melting p-chloronitrobenzene in a melting kettle, and preparing a sodium hydroxide solution in a dissolving kettle; b) repeatedly introducing nitrogen into a system 3-6 times to replace air before a reaction, pumping the heated and molten p-chloronitrobenzene and the sodium hydroxide solution into a micro-reactor by using a feeding valve, uniformly mixing, and carrying out a high-temperature and high-pressure reaction; and c) transferring the solution after the reaction to a cooling crystallization kettle, and cooling to separate out a sodium p-nitrophenolate solid containing crystal water. According to the invention, a hydrolysis reaction is carriedout by adopting a micro-channel, so that the instant uniform mixing and the efficient heat transfer of the raw materials are realized while the equivalence ratio of the materials in the reaction process is reduced, the generation of side reactions and the difficulty in post-treatment are reduced, water can be recycled so as to reduce the energy consumption in production, improve the utilization rate of the raw materials and achieve the environment-friendly production process, the production cost is low, and the reaction time is greatly shortened; and the color of the prepared sodium p-nitrophenolate is faint yellow or orange yellow, and by-products are few.
Owner:珠海派锐尔新材料有限公司
Modification method for raney nickel catalyst for p-chloronitrobenzene hydrogenation
InactiveCN102527393AEasy to solveReduce post-processingOrganic compound preparationAmino compound preparationP-chloroanilineGeneration rate
The invention relates to a modification method for a raney nickel catalyst for p-chloronitrobenzene hydrogenation, and belongs to the technical field of organic synthesis. According to the method, a catalyst is added to deionized water containing 1-5% of a modifier, a stirring reaction is performed for 20-40 minutes at a temperature of 30-70 DEG C, and a standing treatment is performed for 30-90 minutes; a reduction agent with the concentration of 5-15% is added, a stirring reaction is performed for 30-60 minutes at the temperature of 40-50 DEG C, and NaHCO3 is adopted to adjust the pH value to 8-10, wherein a mass ratio of the reduction agent to the raney nickel catalyst is 4:1-8:1; a standing treatment is performed for 30-60 minutes, and deionized water is adopted to wash until the solution is neutral, a filtering treatment is performed, water is added, and sealed preservation is performed to prepare the modified raney nickel catalyst. According to the present invention, under the condition of no addition of the dechlorination inhibitor, the p-chloronitrobenzene conversion rate is more than or equal to 99.9%, the dechlorination side reaction generation rate in the p-chloronitrobenzene hydrogenation process is less than or equal to 2%, and the yield of p-chloroaniline is more than or equal to 95%; compared to the existing methods for preparing the hydrogenation catalyst, the method of the present invention has the following advantages that: the hydrogenation process is simplified, the three waste is less after the hydrogenation, the cost is low, the energy consumption is low, and the industrial requirements of quality increasing, consumption reducing, and environmental protection are achieved.
Owner:CHINA PETROLEUM & CHEM CORP +1
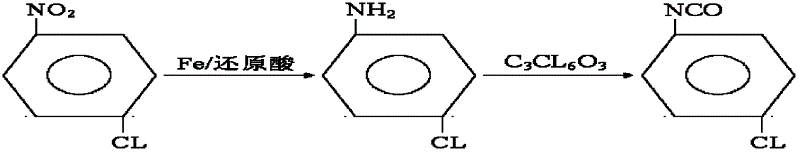
Synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate
InactiveCN102617401AAvoid pollutionAvoid poisoningIsocyanic acid derivatives preparationOrganic compound preparationP-chloroanilineReflux
The invention relates to a synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate. The method comprises the following steps of: reducing parachloronitrobenzene by using iron powder, separating water, dehydrating, adding a solvent, reacting with a bis(trichloromethyl) carbonate solution, distilling to recover the solvent, and distilling to obtain a p-chlorophenol isocyanate finished product. The method has the advantages that a process is advanced; process conditions are reasonable; the production of p-chloroaniline is organically combined with that of p-chlorophenol isocyanate; the environment pollution and an intoxicating phenomenon which are brought by p-chloroaniline used as an industrial product in the processes of aftertreatment, conveying, carrying and feeding can be avoided; virulent phosgene and diphosgene are avoided during production of p-chlorophenol isocyanate, and production is safe and reliable; the residual bis(trichloromethyl) carbonate is removed by normal-pressure reflux; the improvement on the quality of a product is facilitated; and the method has great implementation value and social and economic benefits.
Owner:象山志华新材料有限公司
Synthesis method of 2-amino-5-chlorobenzophenone
InactiveCN107827763AHigh yieldEmission reductionOrganic chemistryOrganic compound preparationPalladium on carbonSynthesis methods
The invention discloses a synthesis method of 2-amino-5-chlorobenzophenone, comprising the following steps: (1) mixing ethanol with a mass concentration of 95%, sodium hydroxide, p-chloronitrobenzene and phenylacetonitrile, Stir, then ultrasonically oscillate at 25-35°C; (2) microwave heating, add water to the system, filter, wash the filter residue with methanol, and then dry to obtain 5-chloro 3-phenyl-2,1 benzo Isoxazole; (3) Mix tetrahydrofuran and triethylamine, then add 5-chloro 3-phenyl-2,1 benzisoxazole and palladium carbon catalyst to mix, pass nitrogen, replace air, and then pass hydrogen To a pressure of 0.1MPa, magnetically stirred, then filtered, and the obtained filter residue was vacuum-dried to obtain 2-amino-5-chlorobenzophenone. The method provided by the invention has high product yield, which can reach 95%, short reaction time, reduced waste discharge, and the production method is environmentally friendly.
Owner:SHAANXI JUJIEHAN CHEM CO LTD
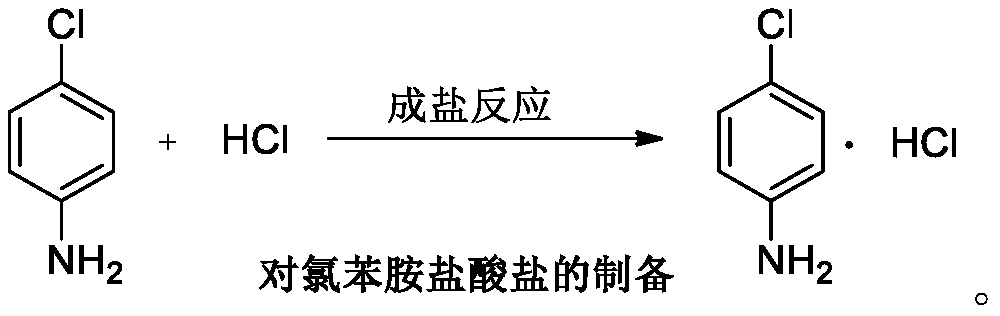
P-chloroaniline hydrochloride preparation method
InactiveCN110467533AModerate reaction conditionsSuppress generationAmino compound purification/separationOrganic compound preparationP-chloroanilineThermal insulation
The invention provides a p-chloroaniline hydrochloride preparation method, which comprises: in the presence of an organic solvent azeotropic with water, using p-chloronitrobenzene as a starting raw material, adding anti-dehalogenation agent and an acid binding agent, sealing, introducing hydrogen gas, carrying out a hydrogenation reaction under the catalysis of a platinum-carbon catalyst at a hightemperature under a high pressure, cooling, filtering to remove the catalyst, collecting the filtrate, adding concentrated hydrochloric acid to the filtrate, carrying out thermal insulation for 0.5-2h at a temperature of 80-95 DEG C, continuously heating to achieve a boiling state, refluxing by a water separator until no water is separated, filtering, and drying to obtain p-chloroaniline hydrochloride. According to the present invention, the preparation method has characteristics of mild and controllable reaction conditions, simple operation and low cost, performs the two steps through the one-pot method, and provides the advanced preparation method for industrial production.
Owner:辽宁顺通化工股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
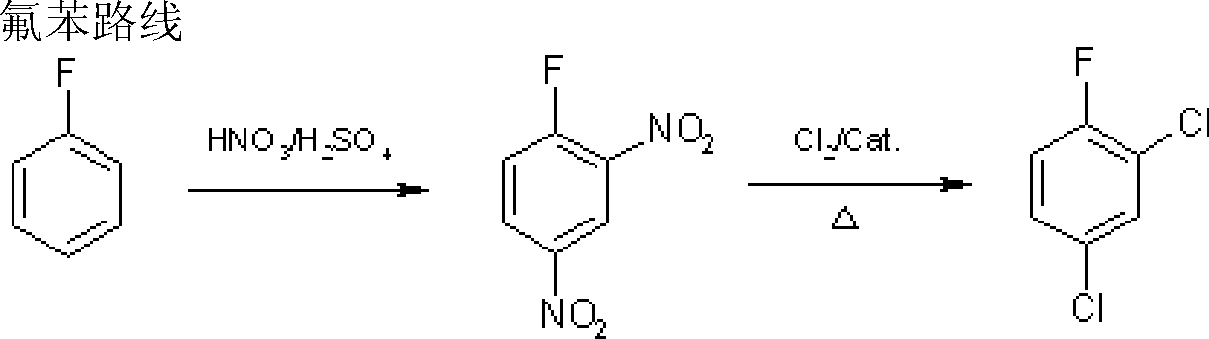

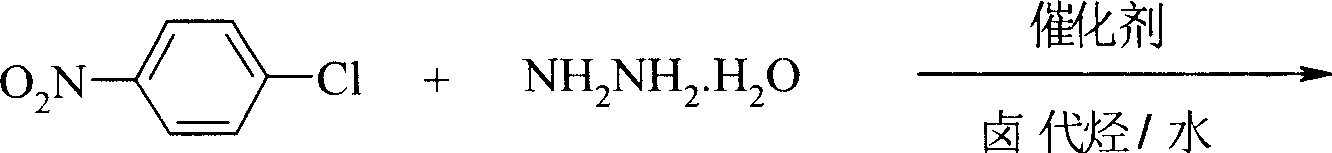
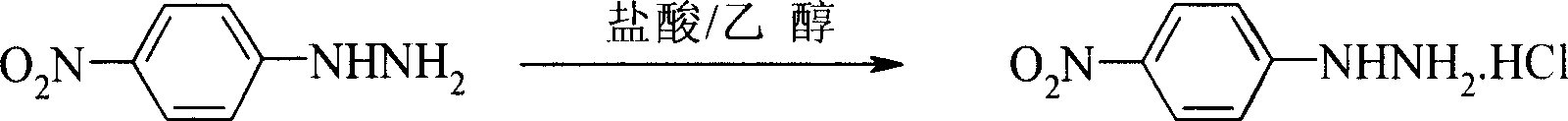

![Preparation method of 2,2-di[4-(4-amidophenoxy)phenyl]hexafluoropropane Preparation method of 2,2-di[4-(4-amidophenoxy)phenyl]hexafluoropropane](https://images-eureka.patsnap.com/patent_img/af7ec0f4-ac9a-4d8a-9954-d951cf17e08c/A20061003001400041.PNG)
![Preparation method of 2,2-di[4-(4-amidophenoxy)phenyl]hexafluoropropane Preparation method of 2,2-di[4-(4-amidophenoxy)phenyl]hexafluoropropane](https://images-eureka.patsnap.com/patent_img/af7ec0f4-ac9a-4d8a-9954-d951cf17e08c/A20061003001400051.PNG)