Preparation method of 2,4-dichloro fluorobenzene
A technology of dichlorofluorobenzene and dichloronitrobenzene, which is applied in the field of fluorobenzene organic compounds, can solve the problems of difficulty and influence in the separation of intermediate fluorochloronitrobenzene isomers, and achieve High product quality, good operating environment and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
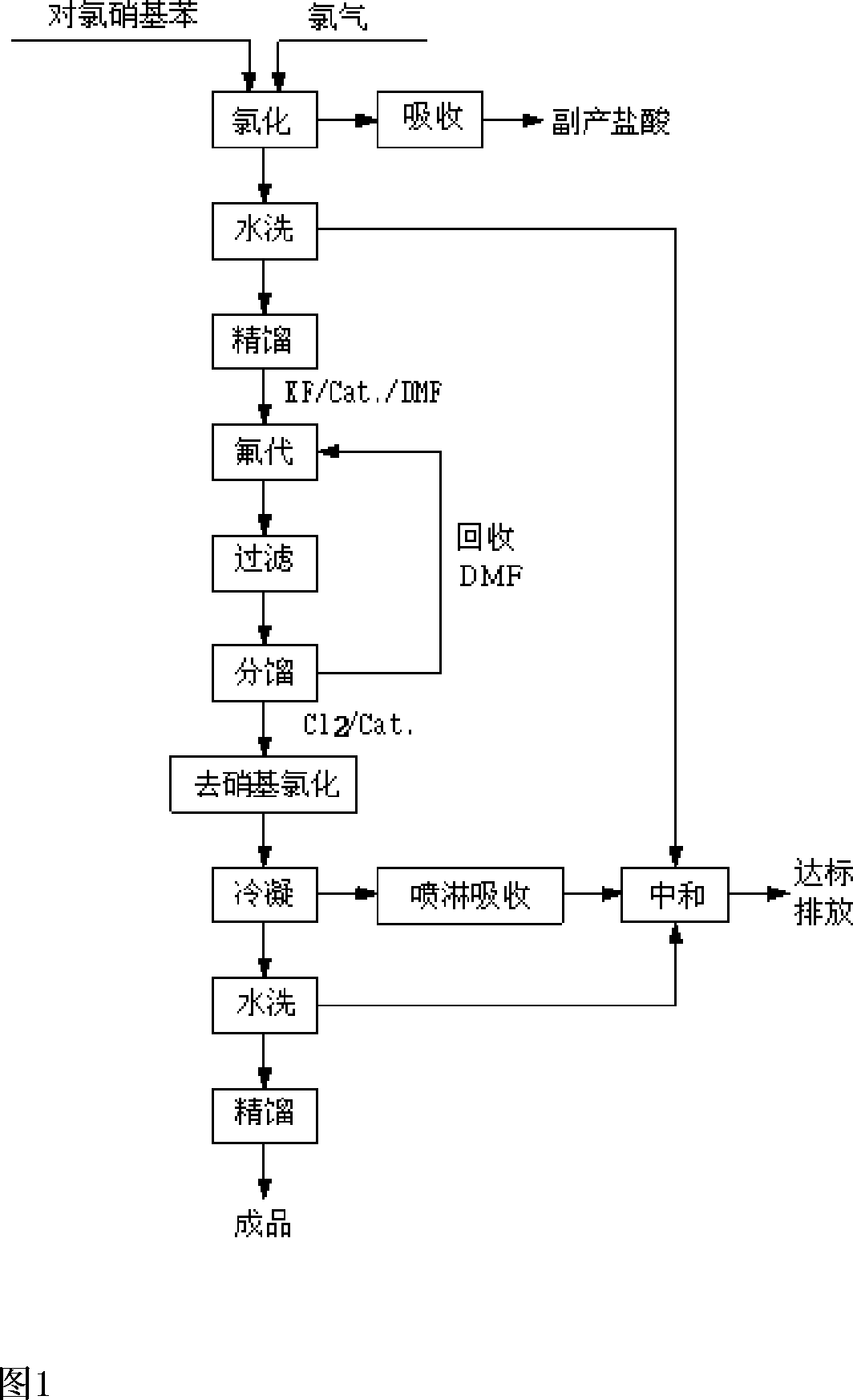
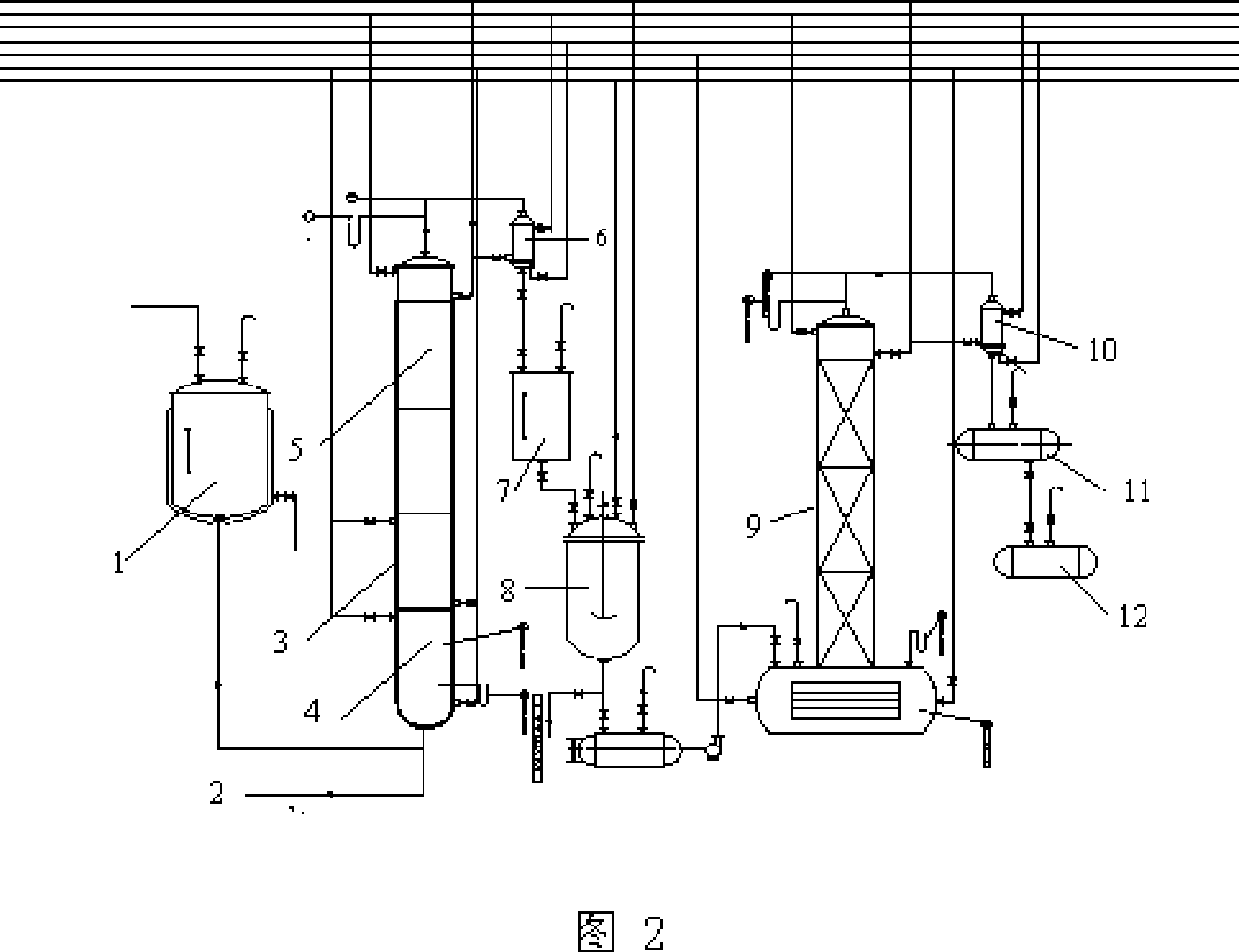
[0032] Embodiments of the present invention: the reaction process of the present invention is as follows:
[0033]
[0034] The whole reaction takes p-chloronitrobenzene as the starting material. Follow the steps below:
[0035] a. Using anhydrous ferric chloride as a catalyst, feed chlorine gas into the reaction kettle to react with p-chloronitrobenzene to generate 3,4-dichloronitrobenzene and hydrogen chloride. Hydrogen chloride is absorbed by water to obtain hydrochloric acid as a by-product. 3,4-Dichloronitrobenzene is washed with water and then rectified for the fluorination reaction in step b.
[0036] B, with 3,4-dichloronitrobenzene in the solvent DMF, the mixture that is 70%~90% with quaternary ammonium salt content and 10%~30% quaternary phosphonium salt content is used as catalyst, and Potassium Fluoride (FK ) generates 3-chloro-4-fluoronitrobenzene and potassium chloride at a temperature of 175-185°C, removes potassium chloride by filtration, and recovers sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com