Method for synthesizing 2,4-dichloroaniline
A technology of dichloroaniline and p-chloronitrobenzene, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve problems such as difficult cost control, reduce raw material consumption and production costs, and reduce reaction side effects The effect of less product and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
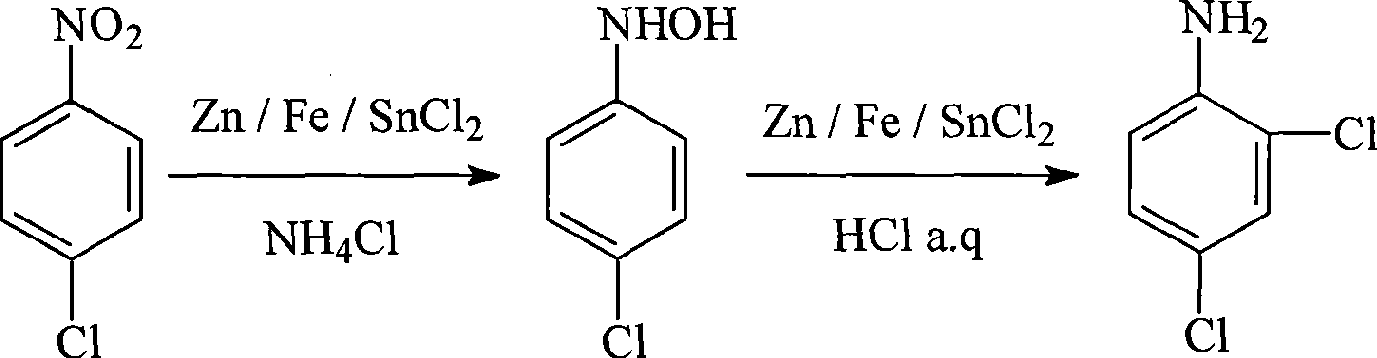
[0032] Add 20ml of ethanol and 6.3g of p-chloronitrobenzene into the 50ml three-necked flask, stir and dissolve, then add 12.6ml of 5% ammonium chloride aqueous solution. Stir and heat under the protection of nitrogen, add 6.5g of zinc powder in batches, control the temperature 75°C during the process of adding zinc powder, and keep stirring at this temperature after the addition is complete until the reaction solution is colorless and transparent.
[0033] After the above reaction solution was cooled to a temperature lower than 60°C, 95 g of concentrated hydrochloric acid was slowly added dropwise under nitrogen protection, while the temperature was controlled at 55-60°C and stirred. After the concentrated hydrochloric acid was added dropwise, the reaction was carried out at 60°C for 1 hour. Cool to room temperature, filter, discard the yellow filter residue, and neutralize the filtrate with ammonia water to pH = 7 under ice-bath conditions, a solid precipitates, and recrysta...
Embodiment 2
[0035] Add 20ml of ethanol and 6.3g of p-chloronitrobenzene into the 50ml three-necked flask, stir and dissolve, then add 12.6ml of 5% ammonium chloride aqueous solution. Stir and heat under the protection of nitrogen, control the temperature at 80°C, add 10g of zinc powder in batches, continue to maintain the temperature after the addition, and stir until the reaction solution is colorless and transparent.
[0036] Filtrate while hot, wash the filter cake with an appropriate amount of hot water, combine the filtrate and washing liquid, add crushed ice and let it stand for cooling to precipitate crystals. The obtained crystals were filtered and washed with petroleum ether to obtain 4.5 g of p-chlorophenhydroxylamine crystals, and the yield of hydroxylamine was 78%.
[0037] Dissolve 4.5g of hydroxylamine crystals in 25ml of ethanol, under nitrogen protection and stirring, use a constant pressure dropping funnel to drop the hydroxylamine ethanol solution into a three-necked bot...
Embodiment 3
[0039] Replace the Zn powder in embodiment 1 with stannous chloride as reducing agent, stannous chloride consumption is 14g, and the charging amount of all the other reagents is all identical with embodiment 1, operates final by the identical reaction condition of embodiment 1 and operating method 5.2 g of 2,4-dichloroaniline was obtained, and the yield of 2,4-dichloroaniline was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com