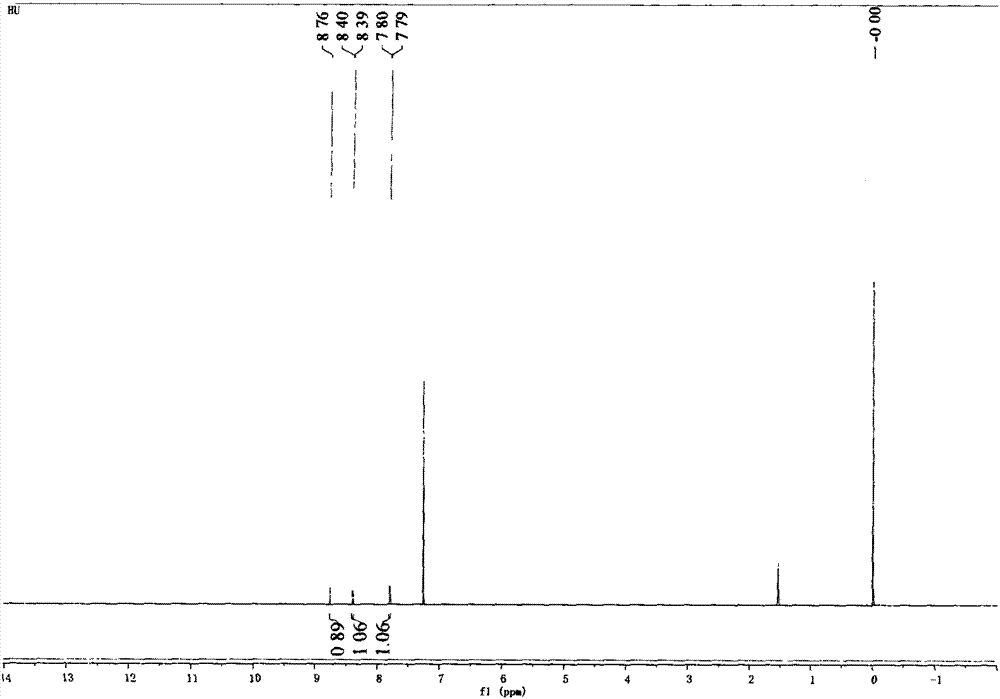
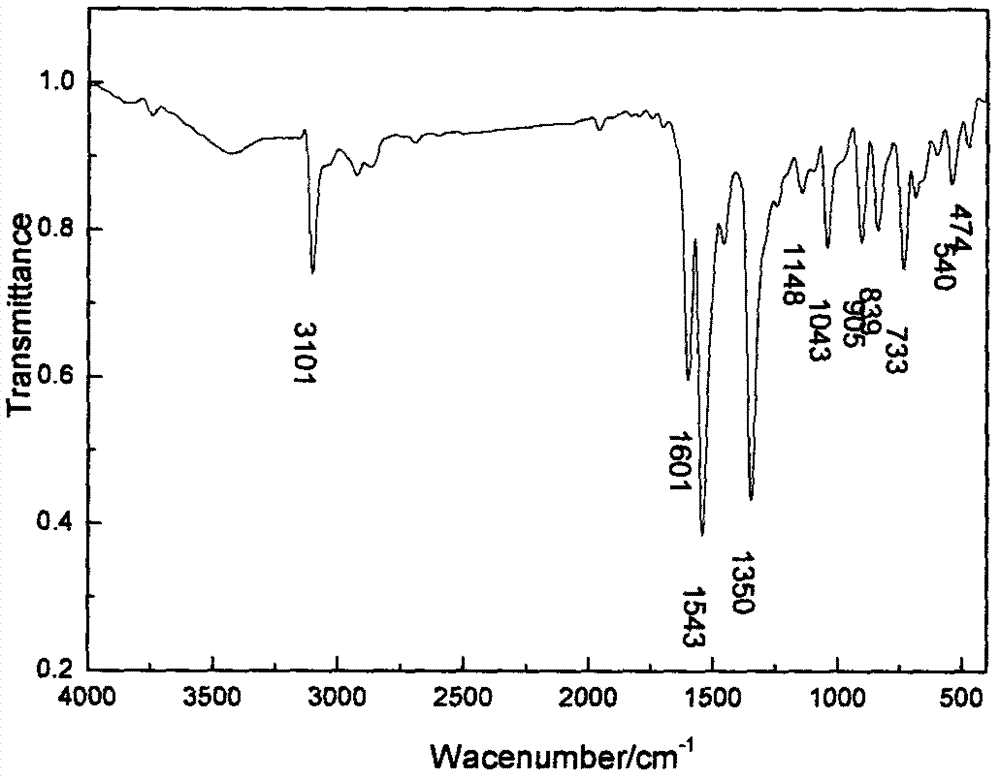
Method for preparing 2, 4-dinitrochlorobenzene by green nitrification technology
A technology of dinitrochlorobenzene and p-chloronitrobenzene is applied in nitrification technology and 2 fields, can solve the problems of high water consumption and energy consumption, difficult process control, slow reaction speed and the like, and achieves low price, simple operation, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Prepare 0.1 g / ml of nitrogen pentoxide / nitric acid (1 gram of nitrogen pentoxide, 10 milliliters of nitric acid) solution in a 100 ml three-necked flask, slowly add 5 grams of p-chloronitrobenzene that have been weighed into the flask, After the addition was complete, the temperature of the water bath was raised to 40°C to start the reaction. Within 100 minutes of the reaction, first take a sample every 5 minutes, take 5 samples, then take a sample every 10 minutes, take 5 samples, and take 10 samples in total. The sample was pipetted for 0.1 minutes of the reaction solution, then 10 ml of ice water was added to terminate the reaction and 10 ml of dichloromethane was used as the extraction agent, neutralized with sodium bicarbonate solution, after separation, 1 ml was added to the organic phase The prepared toluene solution was used as an internal standard, and the concentration of p-chloronitrobenzene in the organic phase was quantitatively analyzed by chromatography u...
example 2
[0018] Prepare 0.1 g / ml of nitrogen pentoxide / nitric acid (1 gram of nitrogen pentoxide, 10 milliliters of nitric acid) solution in a 100 ml three-necked flask, slowly add 5 grams of p-chloronitrobenzene that have been weighed into the flask, After the addition, react at 20°C, 30°C, 40°C, 50°C, and 60°C for 80 minutes respectively, and finally use the internal standard curve method for chromatographic quantitative analysis of the amount of the product in the organic phase.
example 3
[0020] Prepare 0.1 g / ml, 0.2 g / ml, 0.3 g / ml, 0.4 g / ml, 0.6 g / ml, 0.8 g / ml of dinitrogen pentoxide / nitric acid solution (10 ml of nitric acid) in a 100 ml three-necked flask In the flask, 5 grams of p-chloronitrobenzene that had been weighed were slowly added into the flask. After the addition, they were reacted at 50° C. for 80 minutes, and finally the internal standard curve method was used for chromatographic quantitative analysis of the amount of the product in the organic phase.
[0021] By above 3 examples, draw nitrogen pentoxide / nitric acid nitration p-nitrochlorobenzene to prepare 2, the optimum reaction condition of 4-dinitrochlorobenzene is: when p-chloronitrobenzene is 5 grams, the reaction time For 70 minutes, the reaction temperature was 50°C, and the nitrogen pentoxide concentration was g / ml (3 grams of nitrogen pentoxide, 10 milliliters of nitric acid). At this moment, the productive rate of 2,4-dinitrochlorobenzene was 96.73%. , 100% pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com