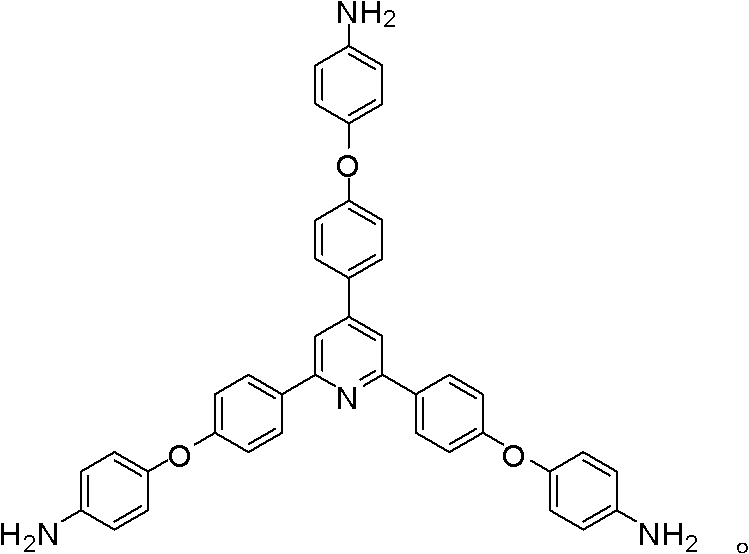
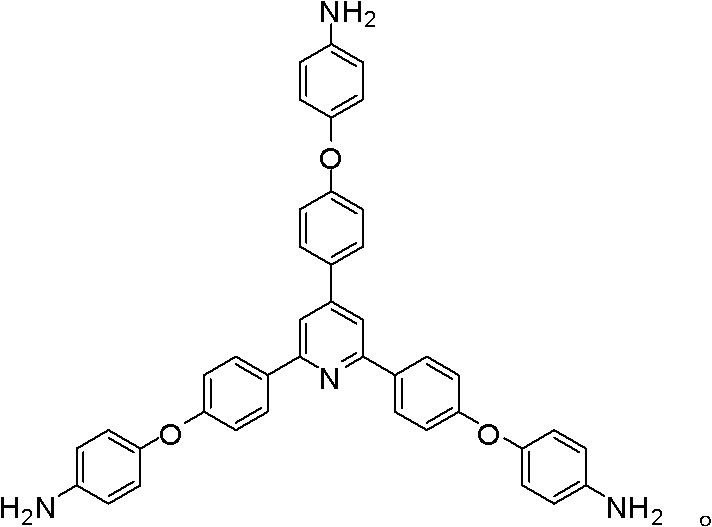
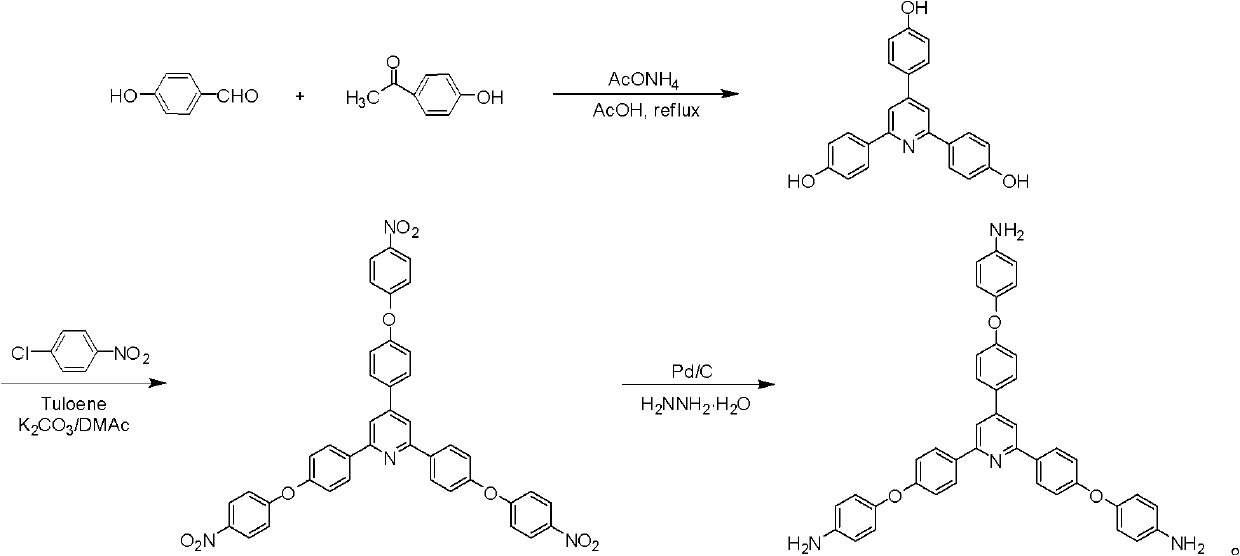
Synthesis of a Triamine Containing a Symmetrical Triarylpyridine Structure and Its Hyperbranched Polyimide
A technology of triarylpyridine and polyimide, which is applied in the field of hyperbranched polyimide polymers, can solve the problems that the product structure is difficult to control, rarely used, and prone to gel.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Add 12.2g (0.1mol) of p-hydroxybenzaldehyde, 27.2g (0.2mol) of p-hydroxyacetophenone, 75g of ammonium acetate and 250mL of glacial acetic acid into a three-necked flask, heat it to reflux for 2 hours after passing nitrogen, and discharge In deionized water, the filtered filter residue was recrystallized by ethanol to obtain a yellow trihydroxypyridine compound 2,4,6-tris(4-hydroxyphenyl)pyridine.
[0032] Yield: 33wt%; FT IR (cm -1 , KBr): 3295 (-OH), 1607, 1548 and 1445 (C=N and C=C on pyridine ring and benzene ring); 1 H NMR (ppm, DMSO-d 6): 9.76 (s, 1H), 9.68 (s, 2H), 8.09-8.08 (d, 4H), 7.83 (s, 2H), 7.80-7.79 (d, 2H), 6.88-6.85 (d, 6H).
[0033] (2) Under nitrogen protection, 8.0g (0.023mol) of the trihydroxypyridine compound obtained by (1), 12.6g (0.080mol) of p-chloronitrobenzene, 11.1g (0.080mol) of potassium carbonate, and N, N- Add 100mL of dimethylacetamide into a three-necked flask, then add 40mL of toluene and raise the temperature to reflux for 15h....
Embodiment 2
[0038] (1) Add 61.1g (0.5mol) of p-hydroxybenzaldehyde, 136.2g (1.0mol) of p-hydroxyacetophenone, 375g of ammonium acetate and 1250mL of glacial acetic acid into a three-necked flask, and heat to reflux for 3 hours after passing through nitrogen, and discharge In deionized water, the filtered filter residue was recrystallized by ethanol to obtain a yellow trihydroxypyridine compound 2,4,6-tris(4-hydroxyphenyl)pyridine.
[0039] Yield: 35wt%; FT IR (cm -1 , KBr): 3297 (-OH), 1605, 1541 and 1439 (C=N and C=C on pyridine ring and benzene ring); 1 H NMR (ppm, DMSO-d 6 ): 9.76 (s, 1H), 9.68 (s, 2H), 8.09-8.07 (d, 4H), 7.83 (s, 2H), 7.79-7.78 (d, 2H), 6.88-6.86 (d, 6H).
[0040] (2) Under nitrogen protection, 40.0g (0.113mol) of the trihydroxypyridine compound obtained by (1), 53.6g (0.340mol) of p-chloronitrobenzene, 47.0g (0.340mol) of potassium carbonate and N, N- Add 500 mL of dimethylacetamide into a three-necked flask, then add 100 mL of toluene and heat up to reflux for 18...
Embodiment 3-8
[0044] The following Examples 3-8 use 2,4,6-tris[4-(4-aminophenoxy)-phenyl]pyridine as a raw material to prepare amino-terminated hyperbranched polyimide polymers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com