Aromatic diamine monomer containing di-tert-butyl structure and preparation method and application of aromatic diamine monomer
An aromatic diamine and tert-butyl technology, which is applied in the field of aromatic diamine monomer and its preparation, can solve the problems of poor dissolving film-forming property and low gas permeability of commercial polyimide, and achieve convenient product separation and synthesis. Effect of simple method, good film formation and gas separation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
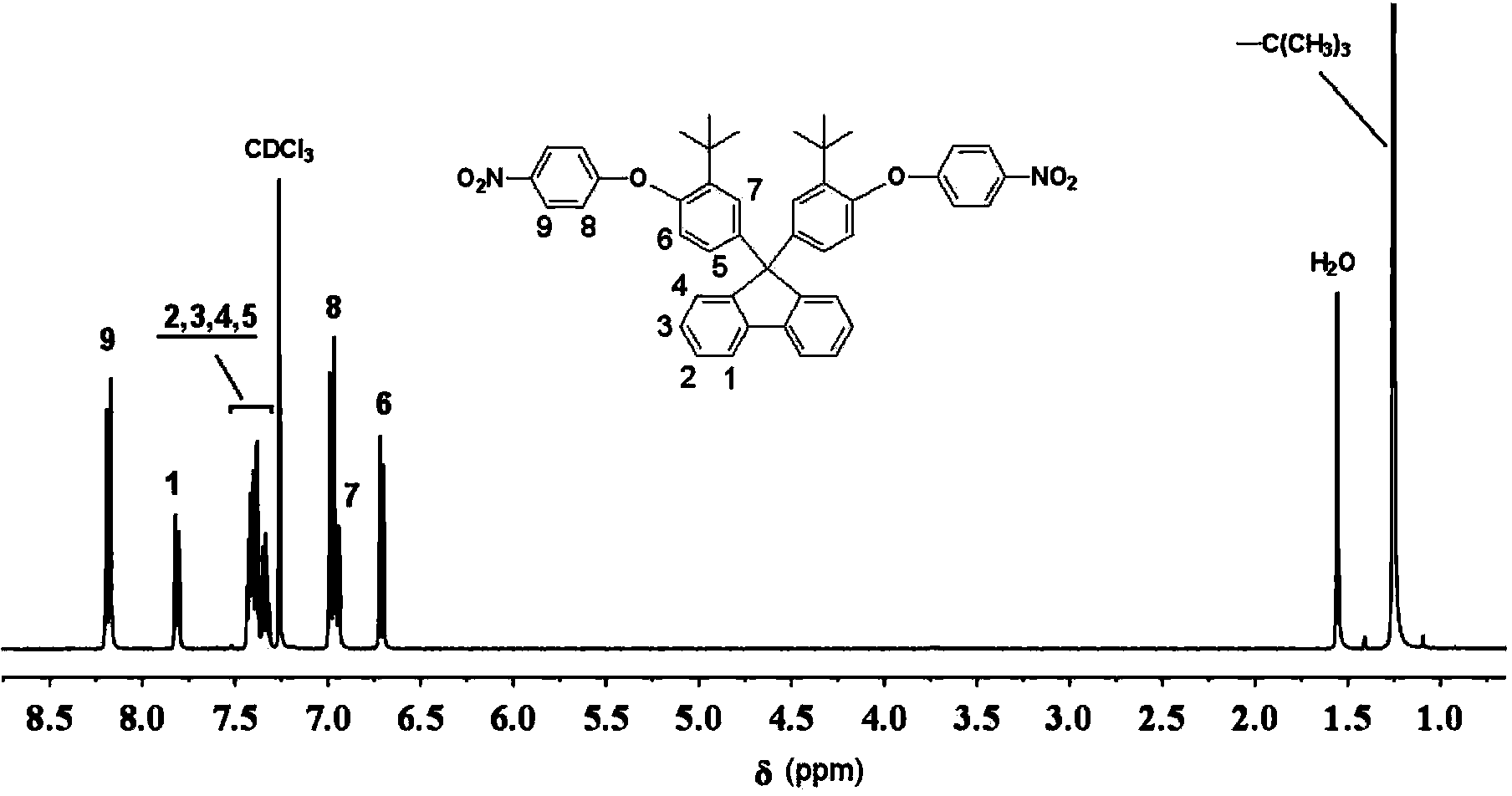
[0030] (1) Add 46.262g (0.1mol) of 9,9-bis(3-tert-butyl-4-hydroxyphenyl)fluorene to a 500ml dry three-necked flask equipped with nitrogen protection, condenser and water separator , 31.51g (0.2mol) of p-chloronitrobenzene, 27.642g (0.2mol) of potassium carbonate, 165ml of N,N-dimethylformamide and 20ml of toluene, after azeotropic dehydration at 150°C for 1 hour, the toluene was distilled off, After continuing to stir and react for 6 hours, cool it and pour it into an ice-water bath, a large amount of solid precipitates out, and is filtered and dried. The crude product is recrystallized with N,N-dimethylformamide to obtain a white crystalline powder which is the intermediate dinitro compound 9. 9-bis[3-tert-butyl-4-(4-nitrophenoxy)phenyl]fluorene, the yield was 82% (as 9,9-bis(3-tert-butyl-4-hydroxyphenyl ) conversion meter of fluorene);
[0031] The melting point of the above-mentioned 9,9-bis[3-tert-butyl-4-(4-nitrophenoxy)phenyl]fluorene is 254~255°C (obtained by DSC test,...
Embodiment 2
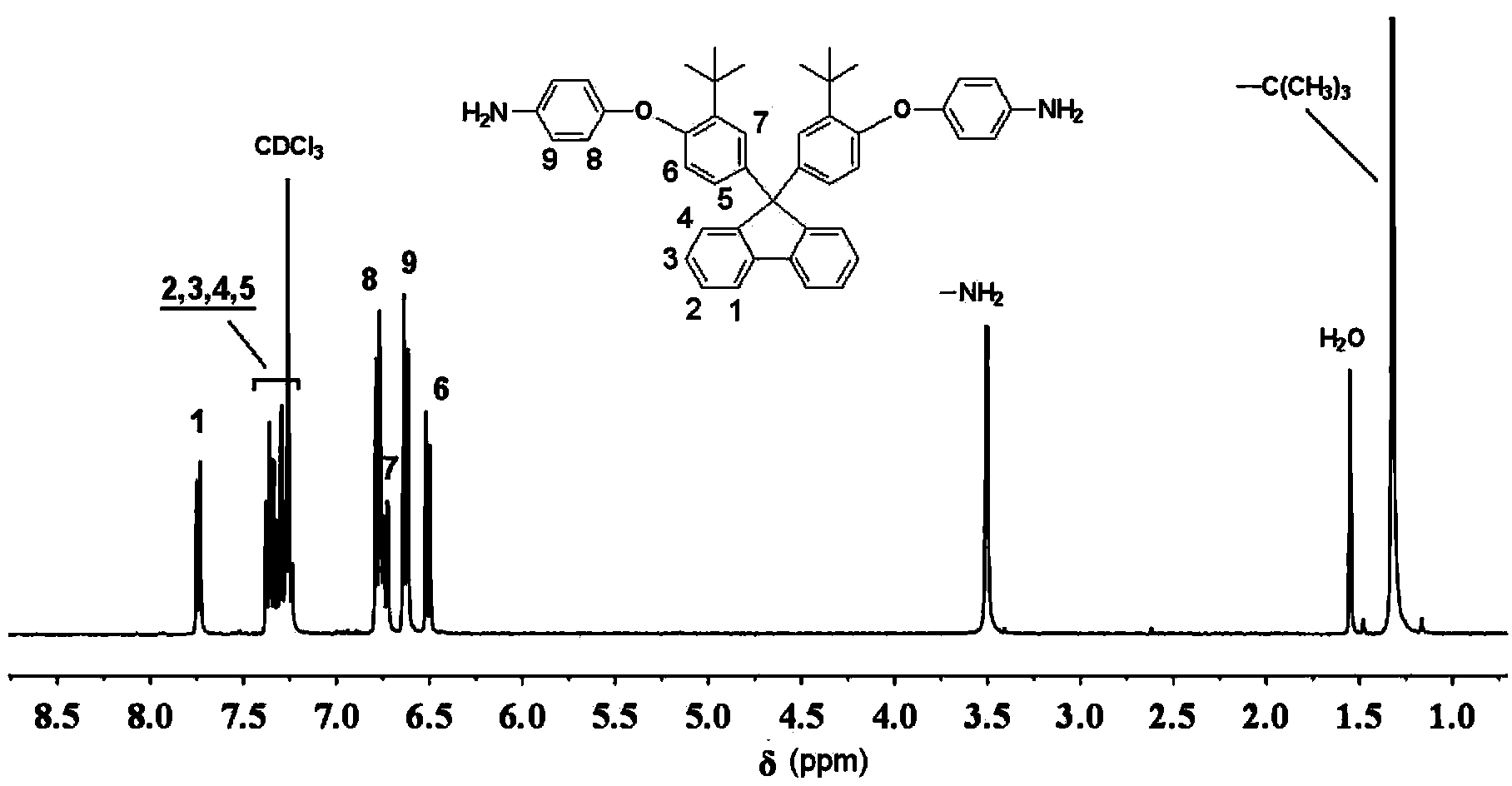
[0035](1) Add 46.262g (0.1mol) of 9,9-bis(3-tert-butyl-4-hydroxyphenyl)fluorene to a 500ml dry three-necked flask equipped with nitrogen protection, condenser and water separator , 36.237g (0.23mol) of p-chloronitrobenzene, 41.463g (0.3mol) of potassium carbonate, 260ml of N,N-dimethylformamide and 78ml of toluene, after azeotropic dehydration at 130°C for 3 hours, the toluene was distilled off, After continuing to stir and react for 10 h, cool it and pour it into an ice-water bath, a large amount of solid precipitates out, and is filtered and dried. The crude product is recrystallized with N,N-dimethylacetamide to obtain a white crystalline powder which is the intermediate dinitro compound 9. 9-bis[3-tert-butyl-4-(4-nitrophenoxy)phenyl]fluorene, the yield was 81% (as 9,9-bis(3-tert-butyl-4-hydroxyphenyl ) fluorene conversion meter).
[0036] (2) In a 500ml three-necked flask, add 35.24g (0.05mol) of the dinitro compound 9,9-bis[3-tert-butyl-4-(4-nitrophenoxy) obtained in ste...
Embodiment 3
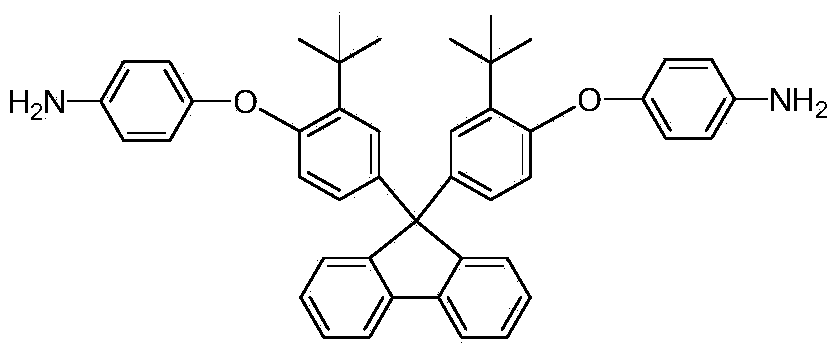
[0039] In a dry 50ml three-necked round-bottomed flask with nitrogen, add 9,9-bis[3-tert-butyl-4-(4-aminophenoxy)phenyl]fluorene and 3mmol obtained in Example 1 3mmol aromatic dianhydride monomer (take diphenyl ether tetra-acid dianhydride as an example), then add 12ml m-cresol and 0.78g isoquinoline respectively, raise the temperature of the reaction system to 180°C and stir for 20h, then finish the reaction and cool to After room temperature, pour the polymer solution into methanol to obtain a fibrous polyimide polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com