Farnesyl acetone and preparation method thereof
A technology of farnesyl acetone and farnesene, which is applied to the preparation of carbon-based compounds, carboxylic acid esters, chemical instruments and methods, etc., can solve the problems of low efficiency, cumbersome post-processing, not enough economical and environmental protection, and meet the reaction conditions Gentle, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 provides a kind of using α-farnesene as raw material, prepares the method for farnesyl acetone, and this method comprises:
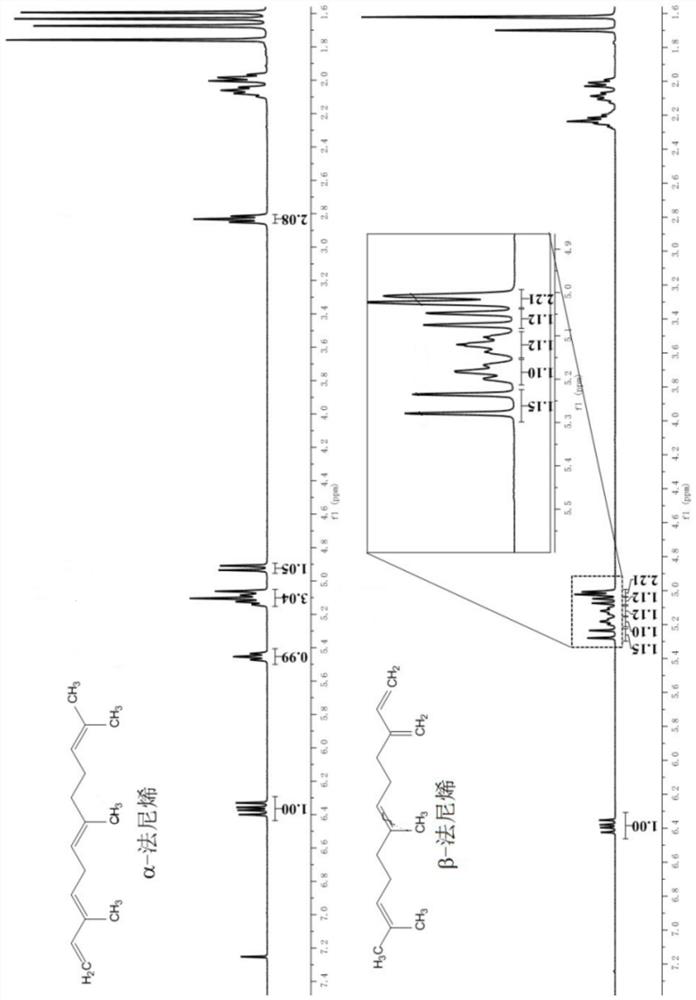
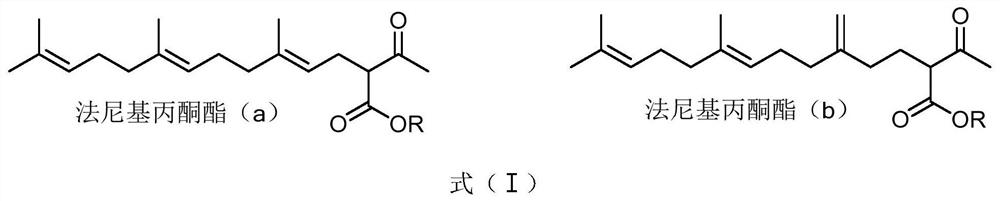
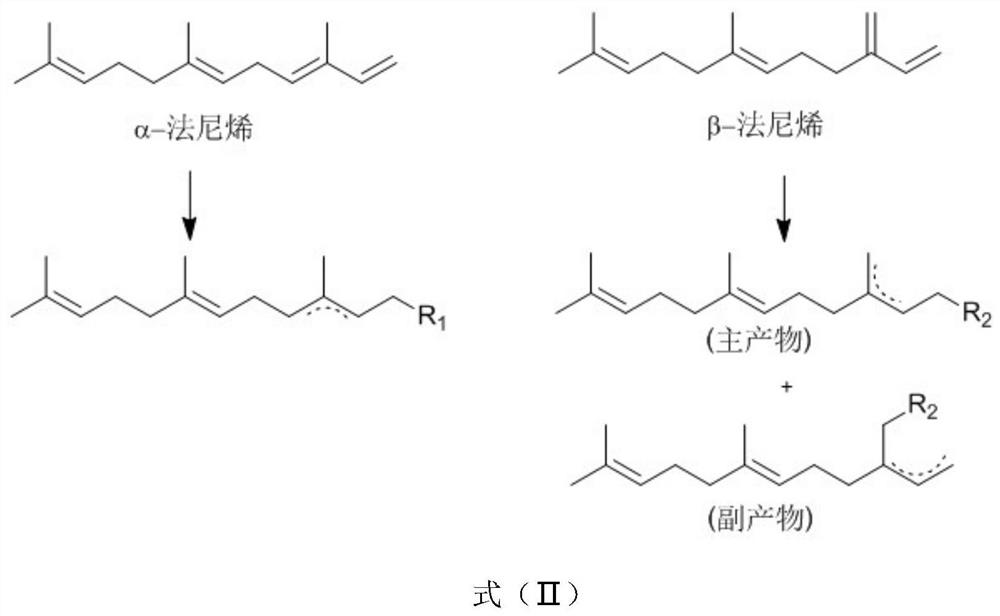
[0058] First, rhodium catalyzes the preparation of farnesyl acetonate, including: α-farnesene (10.3g, 98%) is placed in a 500ml reactor, and 100mL of ethanol, 20mL of deionized water, and triphenylphosphine are added to the system. Sodium trimetasulfonate (600mg) and bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate (50mg) were added to ethyl acetoacetate and heated to 100°C, and refluxed for 10h. Static layered filtration, the reaction solution was concentrated under reduced pressure, extracted with ethyl acetate, concentrated by distillation under reduced pressure to obtain 16.52 g of farnesylacetonate, and the yield of farnesylacetonate was determined by the above-mentioned gas chromatography to be 98.20%.
[0059] Wherein, the output of farnesyl acetonate is calculated and obtained by the following method:
[0060] The ...
Embodiment 2
[0066] Embodiment 2 provides a kind of method that takes α-farnesene as raw material to prepare farnesyl acetonate, in the process of preparing farnesyl acetonate with α-farnesene as raw material, utilize respectively in embodiment 2 and The different rhodium catalysts of embodiment 1 prepare farnesyl acetonate, and other used reaction conditions and raw materials are all identical with embodiment 1, and its experimental result is as shown in table 1 below.
[0067] The impact of different rhodium metal catalysts on the reaction of table 1
[0068]
[0069] It can be seen from the above experimental groups that the yield of farnesyl acetonate is at least 97% or more, and some even reach more than 98% by using different rhodium metal catalysts to prepare farnesyl acetonate.
Embodiment 3
[0071] Embodiment 3 provides a kind of method for preparing farnesyl acetonate with α-farnesene as raw material, in the process of preparing farnesyl acetonate with α-farnesene as raw material, studied respectively in embodiment 3 Catalyzed reaction at different temperatures was used to prepare farnesyl acetonate. Other reaction conditions and raw materials used were the same as in Example 1. The experimental results are shown in Table 2.
[0072] The impact of table 2 different temperatures on farnesyl acetonate output
[0073] Numbering Reaction temperature (°C) Yield of farnesyl acetonate (%) Experimental group 1 80 95.31 Experimental group 2 90 97.56 Experimental group 3 100 98.87 Experimental group 4 110 97.35 Experimental group 5 120 97.10
[0074] From the results of the above examples, it can be seen that in the process of preparing farnesylacetonate from α-farnesene, the yield of farnesylacetonate obtained is slight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com