Co-crystal forms of a novobiocin analog and proline
A co-crystal and proline technology, applied in the direction of sugar derivatives, sugar derivatives, sugar derivatives preparation, etc., can solve the problem that the exact role has not been fully characterized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Example 1: Synthesis of 4a / L-proline co-crystal (Material A)
[0164]
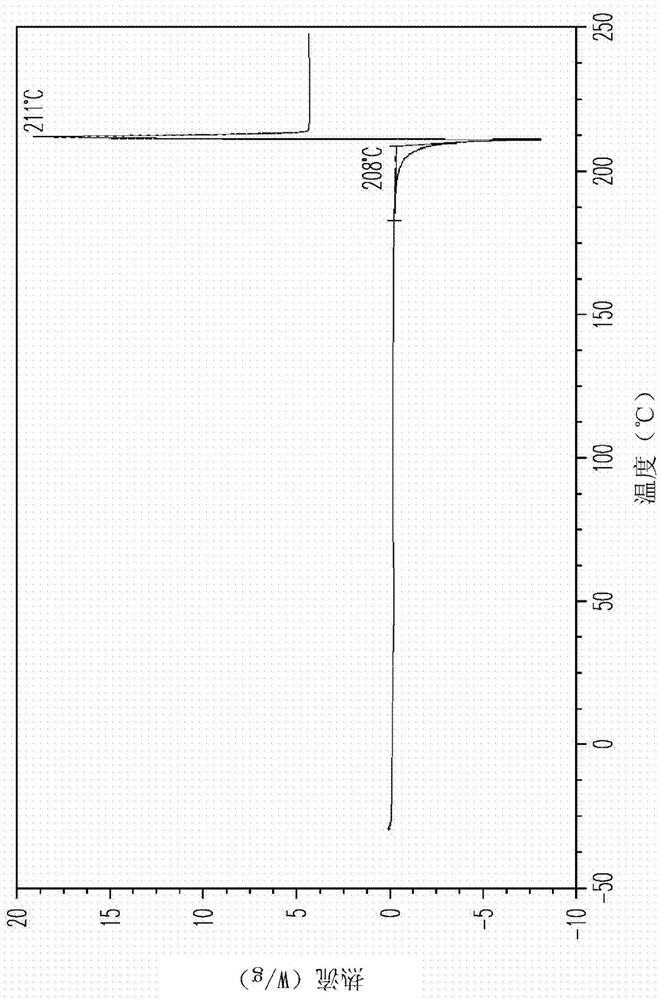
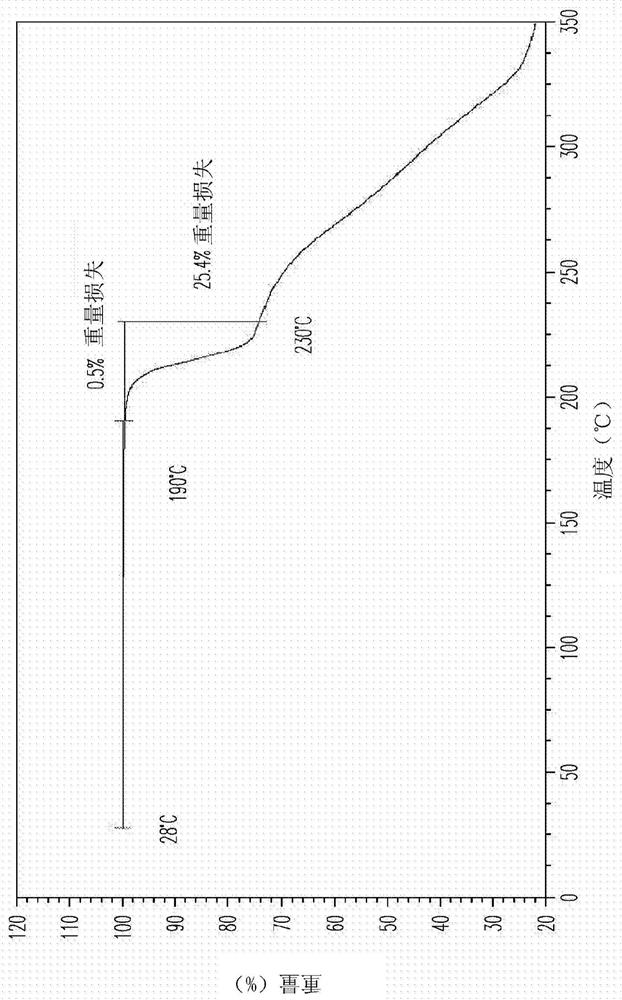
[0165]Compound 4a was obtained by chromatography (HPLC: 4a, 96.4% and 4b, 1.2%, 500 mg) and mixed with L-proline (128 mg, 1 equiv) in EtOH (4 mL). The mixture was heated to reflux for 15 minutes. The hot solution is filtered through a cotton plug. The resulting clear filtrate was cooled slowly and kept at room temperature for 16 hours. The precipitated solid was collected by filtration and dried in air at room temperature to give 4a / L-proline co-crystal designated as Material A (456 mg, 73% yield) as a white solid. M.P. 203-205°C. 1 H NMR showed a 1:1.1 ratio of 4a to L-proline.
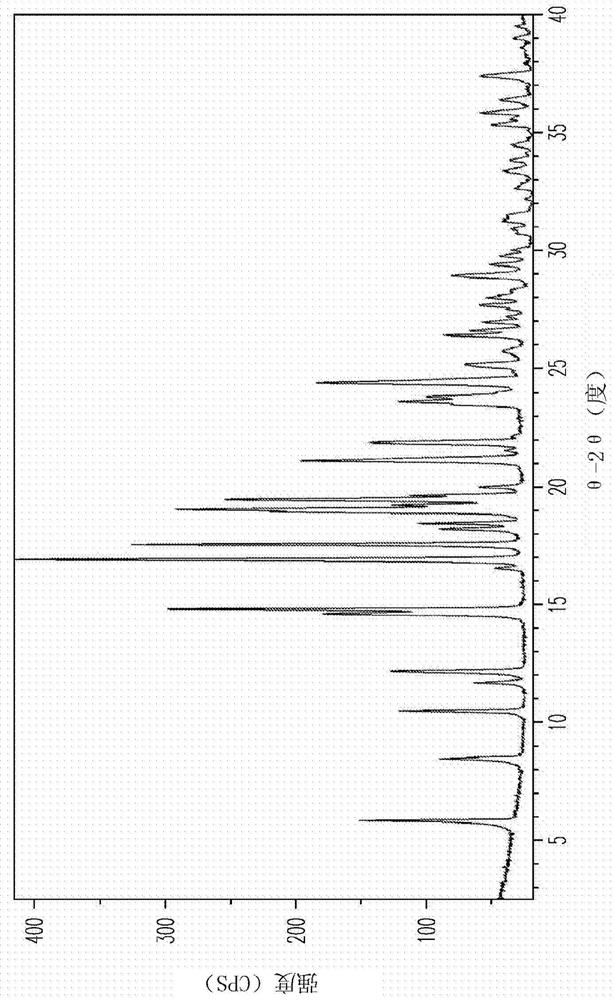
[0166] Material A is a 1:1 4a / L proline co-crystal, and it may be an isostructural solvate. Material A contains less L-proline components based on XRPD patterns ( Figure 23 ), the components have been successfully indexed (Table A1). For samples consisting mainly or only of a single crystalline phase, XRPD index...
Embodiment 2
[0175] Example 2: Purification of 4a by co-crystallization with L-proline
[0176] 500 mg of a mixture 4 consisting of compounds 4a and 4b (HPLC: 4a 92.0% and 4b 7.1%) and L-proline (128 mg, 1 equiv) in ethanol (4 mL) was refluxed for 15 minutes. The mixture was seeded with the co-crystal obtained in Example 1, and the mixture was allowed to cool and then kept at room temperature for 18 hours. The white solid was filtered off while the remaining solid was removed from the reaction flask along with the mother liquor. The amount of collected 4a / L-proline co-crystal (by 1 1:1 ratio determined by H-NMR, 497 mg, 79% yield) consisted of 98.0% 4a and 1.49% 4b (analysis by HPLC).
Embodiment 3
[0177] Example 3: Co-crystal Screening
[0178] Amorphous 4a was used in approximately 50 co-crystal screening experiments with 26 coformers other than L-proline and D-proline, as shown in Table 1 below. A variety of crystallization techniques suitable for co-crystal formation have been used, including solvent-assisted and hand milling, cooling, evaporation, slurry, disintegrating precipitation, and reactive crystallization, in which a solution containing a high molar excess of one component is mixed with the other One component combines to promote reaction equilibrium to favor co-crystal formation. A variety of coformers with functional groups capable of hydrogen bonding have been used, including carboxylic acids, amino acids, sugars, amides, amines, and many functional aromatic compounds. However, 4a did not form any confirmed co-crystals with these common co-formers under the various conditions and co-formers studied in this screen.
[0179] Table 1
[0180]
[0181] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com