Synthesis method of 3', 5'-dichloro-2, 2, 2-trifluoroacetophenone
A technology of trifluoroacetophenone and synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of extremely high requirements on industrial equipment, unsuitable for industrial production, and high industrial production costs , to achieve the effect of simplifying the production separation process, low price and reducing cost consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
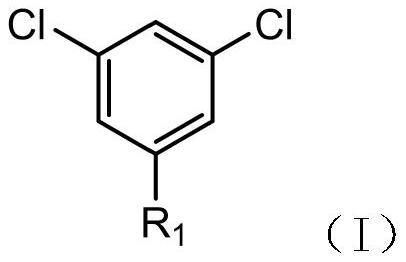
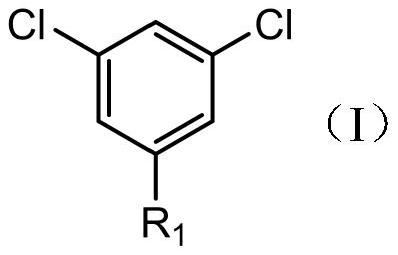
[0039] A kind of synthetic method of 3',5'-dichloro-2,2,2-trifluoroacetophenone, using compound I as raw material, prepared through the following steps:
[0040] S1, react compound I with magnesium to obtain the Grignard reagent of compound I;
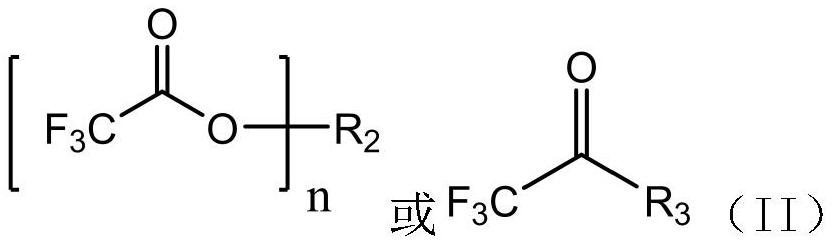
[0041] S2. React the Grignard reagent of compound I obtained in step S1 with compound II, and then treat with acid to obtain 3',5'-dichloro-2,2,2-trifluoroacetophenone.
[0042]In the above reaction, compound I is 1,3,5-trichlorobenzene, and compound II is trifluoroacetyldimethylamine.
[0043] Step S1 is specifically as follows:
[0044] S1-1. Weigh 7.2g of magnesium chips (0.3mol) into the reaction flask, add 60mL of tetrahydrofuran (solvent I), and stir evenly at room temperature to obtain the mixed system I;
[0045] S1-2. Weigh 34.4g of 1,3,5-trichlorobenzene and dissolve it with 90mL of tetrahydrofuran (solvent I) to obtain the mixed system II, and put the mixed system II in a separatory funnel for later use;
[0046] S1-3. Ra...
Embodiment 2
[0051] A method for synthesizing 3',5'-dichloro-2,2,2-trifluoroacetophenone, the difference from Example 1 is that in step S2, compound II uses the amount of trifluoroacetyl di Ethylamine (55.8 g) was substituted for trifluoroacetyldimethylamine.
Embodiment 3
[0053] A synthetic method of 3',5'-dichloro-2,2,2-trifluoroacetophenone, the difference from Example 1 is that in step S2, compound II uses trifluoroacetylpiper Pyridine (52.3 g) was substituted for trifluoroacetyldimethylamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com