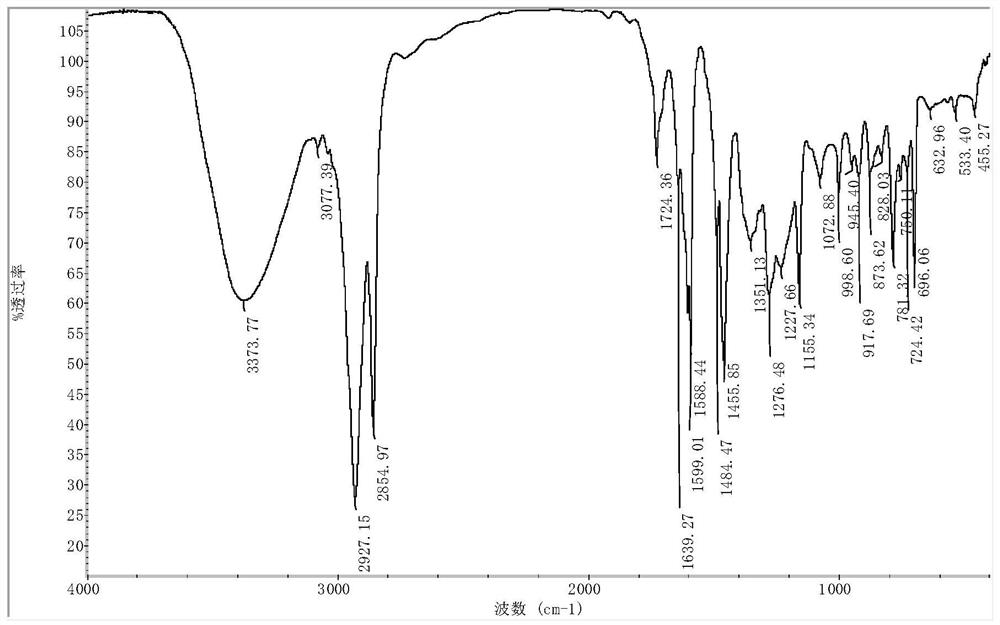
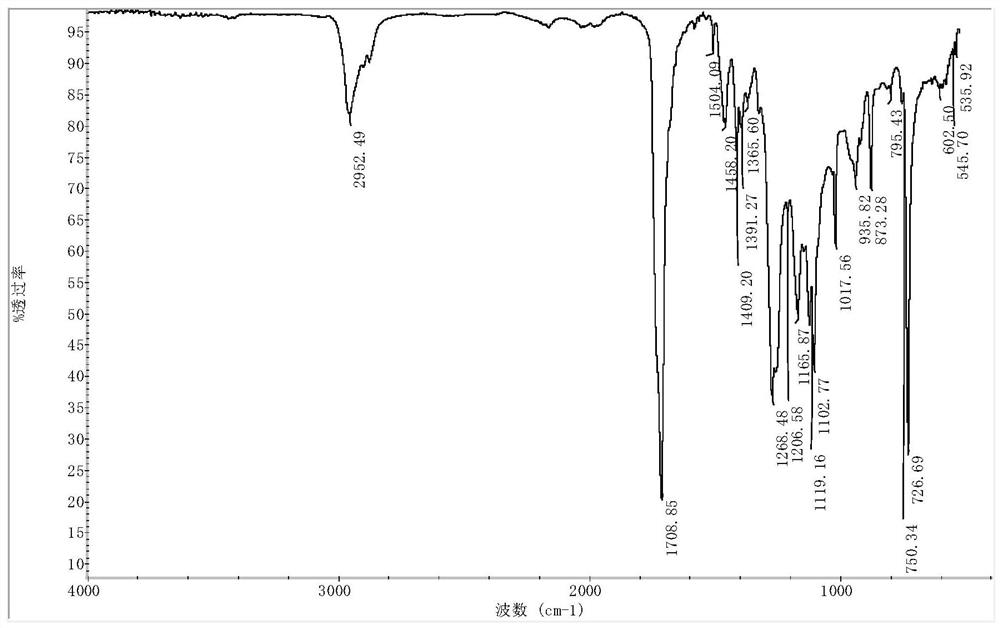
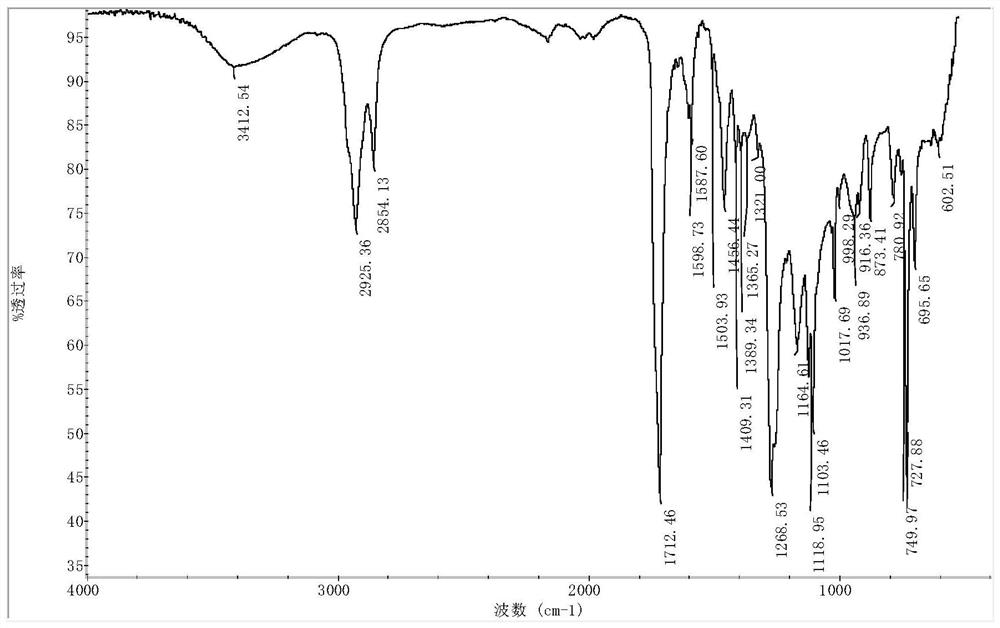
Synthesis method of poly polyethylene terephthalate-adipate-m-epoxypentadecyl phenol butanediete
A technology of epoxy pentadecylphenol butylene glycol and m-epoxypentadecylphenol, which is applied in the field of polymer material synthesis, can solve the problem of high price, poor aging resistance of PBAT film, and unsatisfactory mechanical properties. Demand and other issues, to achieve the effects of improving aging resistance, reducing application costs, and reducing the content of terminal carboxyl groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of synthetic method of polyterephthalic acid-adipic acid-m-epoxypentadecylphenol butylene glycol ester, comprises the following steps:
[0034] 1) Synthesis of m-epoxypentadecylphenol
[0035] Add 500g of pentadecenylphenol and 50g of formic acid into a three-necked round-bottomed flask with mechanical stirring, a separatory funnel and a thermometer, in 390g of 50% H 2 o 2 Add 5g of phosphoric acid into the separatory funnel and start dropping. The dropping temperature does not exceed 40°C. After the dropping is completed, the temperature is raised to 60°C to continue the reaction for 3 hours. , the upper layer oily ester is washed to neutrality, and 50 ℃ dewaters through vacuum distillation to obtain light yellow oily matter m-epoxypentadecylphenol, its epoxy value is 4.52%, and the viscosity at 25 ℃ is 8250cps;
[0036] 2) Synthesis of polyadipate / butylene terephthalate (PBAT)
[0037] Using the co-esterification method, using n-tetrabutyl titanate as a cata...
Embodiment 2
[0041] A kind of synthetic method of polyterephthalic acid-adipic acid-m-epoxypentadecylphenol butylene glycol ester, comprises the following steps:
[0042] 1) Preparation of m-epoxypentadecylphenol
[0043] Add 500g of pentadecenylphenol and 70g of formic acid into a three-necked round-bottomed flask with mechanical stirring, a separatory funnel and a thermometer, in 350g of 50% H 2 o 2Add 8g of phosphoric acid into the separatory funnel and start dropping. The dropping temperature does not exceed 40°C. After the dropping is completed, the temperature is raised to 55°C to continue the reaction for 3.5h. Water, and the upper layer of oil and ester was washed with water until neutral, and the water was removed by distillation under reduced pressure at 55°C to obtain a light yellow oily substance, m-epoxypentadecylphenol, with an epoxy value of 4.47%, and a viscosity of 8968cps at 25°C;
[0044] 2) Synthesis of polyadipate / butylene terephthalate (PBAT)
[0045] Using the co-...
Embodiment 3
[0049] A kind of synthetic method of polyterephthalic acid-adipic acid-m-epoxypentadecylphenol butylene glycol ester, comprises the following steps:
[0050] 1) Preparation of m-epoxypentadecylphenol
[0051] Add 500g of pentadecenylphenol and 100g of formic acid into a three-necked round-bottomed flask with mechanical stirring, a separatory funnel and a thermometer, in 300g of 50% H 2 o 2 Add 10g of phosphoric acid into the separatory funnel and start dropping. The dropping temperature does not exceed 40°C. After the dropping is completed, the temperature is raised to 50°C to continue the reaction for 4 hours. , the upper layer of oily ester was washed to neutrality, and 60 DEG C dewatered under reduced pressure to obtain light yellow oily matter meta-epoxypentadecylphenol, its epoxy value was 4.31%, and its viscosity at 25 DEG C was 9525 cps;
[0052] 2) Synthesis of polyadipate / butylene terephthalate (PBAT)
[0053] Using the co-esterification method, using n-tetrabutyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com