A kind of preparation method of zeolite imidazole frame nanoparticle material loaded with zoledronic acid
A technology of zoledronic acid and zeolite imidazole, which is applied in the field of preparation of zeolite imidazole framework nanoparticle materials, can solve the problems of affecting the overall performance of nanomaterials, limiting the pharmacological activity of zoledronic acid, and being unable to uniformly load zoledronic acid, etc. , to achieve good drug loading capacity, good biological safety, and improve the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for preparing a zoledronic acid-loaded zeolite imidazole framework nanoparticle material specifically targeting bone tissue, comprising the following steps:
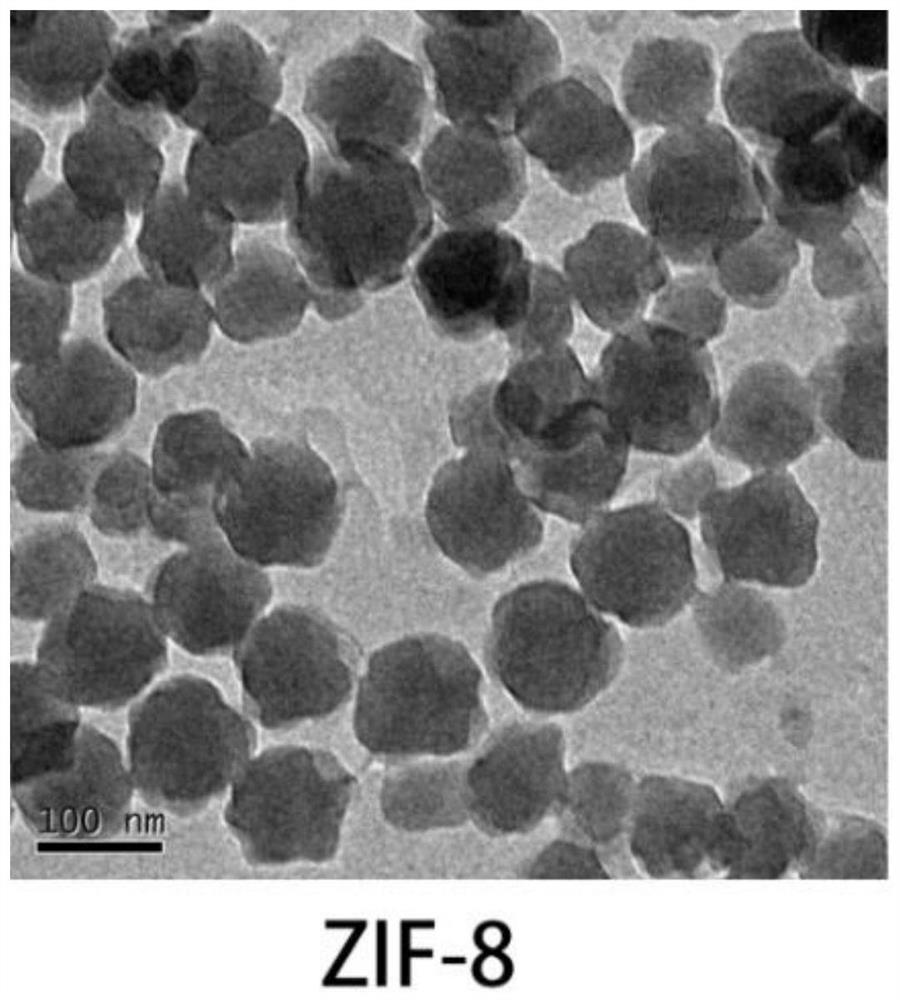
[0040] (1) Prepare 9mL of 2-methylimidazole aqueous solution (pH=8.5) with a concentration of 0.04mol / L, and slowly add Zn(NO 3 ) 2 Aqueous solution (pH = 7) 1mL, continue to stir for 30min, until the reaction system is white colloid, this time is ZIF-8 nanoparticle colloid;
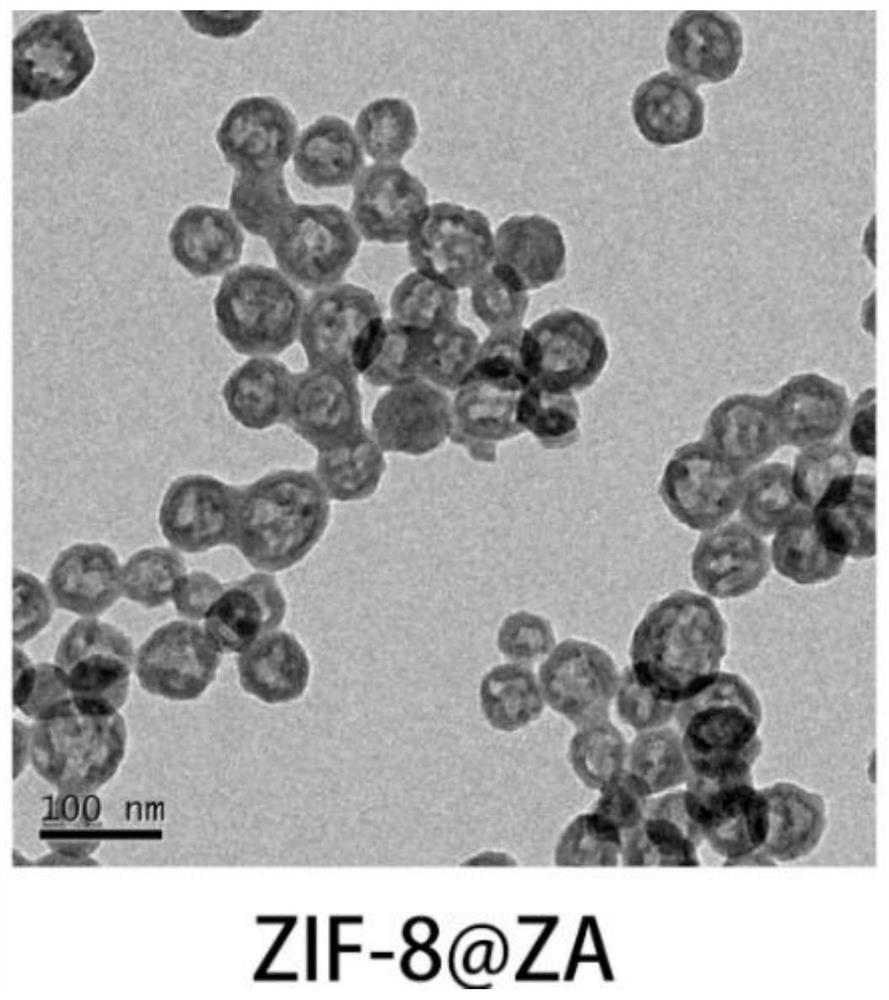
[0041] (2) In the zeolite imidazole framework-8 colloid obtained in step (1), add dropwise 0.6 mL of zoledronic acid aqueous solution (pH=7) with a concentration of 0.05 mol / L while stirring, and continue stirring for 30 min, 10000 g / 10 min Centrifugation and deionized water washing were repeated three times, and the obtained white precipitate was the zeolitic imidazole framework nanoparticle material (ZIF-8@ZA nanoparticles) loaded with zoledronic acid. After freeze-drying, the yield was weighed to 10 mg.
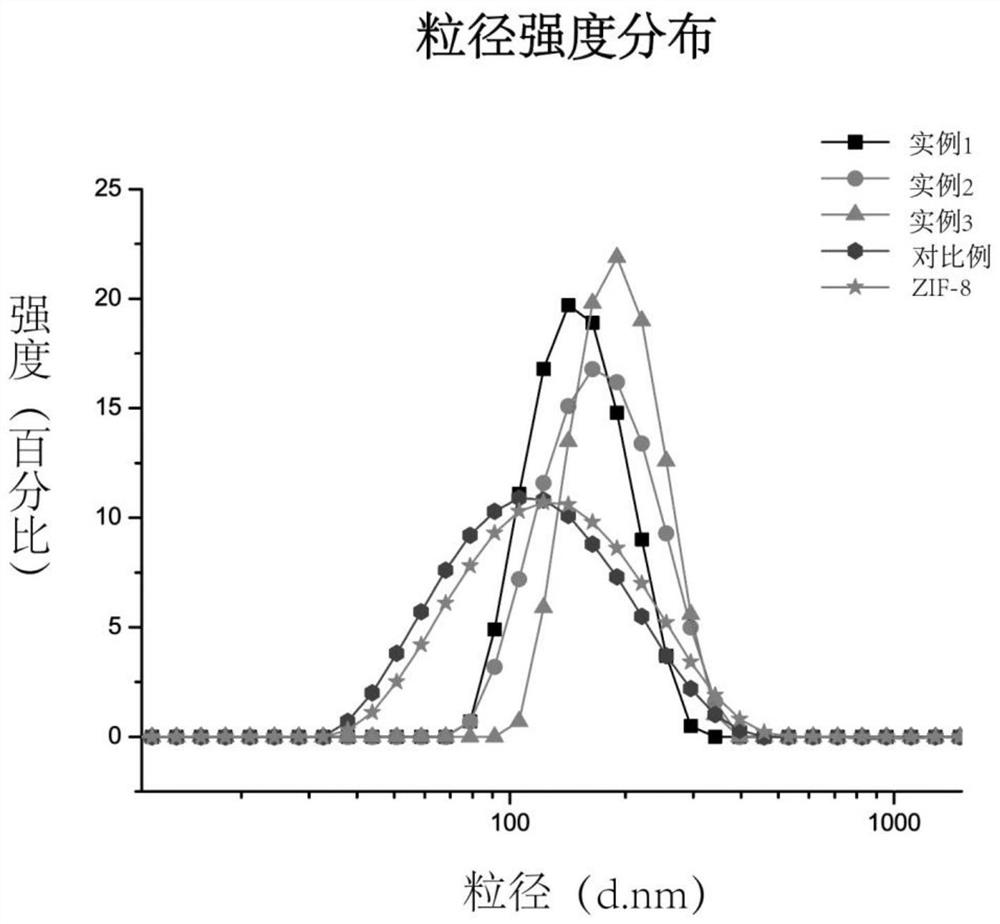
[0042] 1. Characteriza...
Embodiment 2
[0056] A method for preparing a zoledronic acid-loaded zeolite imidazole framework nanoparticle material specifically targeting bone tissue, comprising the following steps:
[0057] (1) Prepare 9mL of 2-methylimidazole aqueous solution (pH=8.5) with a concentration of 0.05mol / L, and slowly add Zn(NO 3 ) 2 Aqueous solution (pH = 7) 1mL, continue to stir for 30min, until the reaction system is white colloid, this time is ZIF-8 nanoparticle colloid;
[0058] (2) In the zeolite imidazole framework-8 colloid obtained in step (1), add dropwise 0.6 mL of zoledronic acid aqueous solution (pH=7) with a concentration of 0.05 mol / L while stirring, and continue stirring for 30 min, 10000 g / 10 min Centrifugation and deionized water washing were repeated three times, and the obtained white precipitate was the zeolite imidazole framework nanoparticle material (ZIF-8@ZA nanoparticles) loaded with zoledronic acid. After freeze-drying, the yield was 8 mg.
Embodiment 3
[0060] A method for preparing a zoledronic acid-loaded zeolite imidazole framework nanoparticle material specifically targeting bone tissue, comprising the following steps:
[0061] (1) Prepare 9mL of 2-methylimidazole aqueous solution (pH=8.5) with a concentration of 0.03mol / L, and slowly add Zn(NO 3 ) 2 Aqueous solution (pH = 7) 1mL, continue to stir for 30min, until the reaction system is white colloid, this time is ZIF-8 nanoparticle colloid;
[0062] (2) Add 0.6 mL of zoledronic acid aqueous solution (pH=7) with a concentration of 0.06 mol / L dropwise to the zeolite imidazole framework-8 colloid obtained in step (1), and continue stirring for 30 min, 10000 g / 10 min Centrifugation and deionized water washing were repeated three times, and the obtained white precipitate was the zeolite imidazole framework nanoparticle material (ZIF-8@ZA nanoparticles) loaded with zoledronic acid. After freeze-drying, the yield was 8 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com