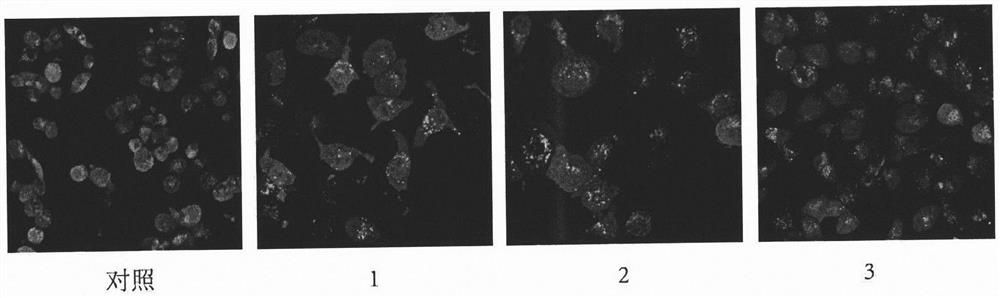
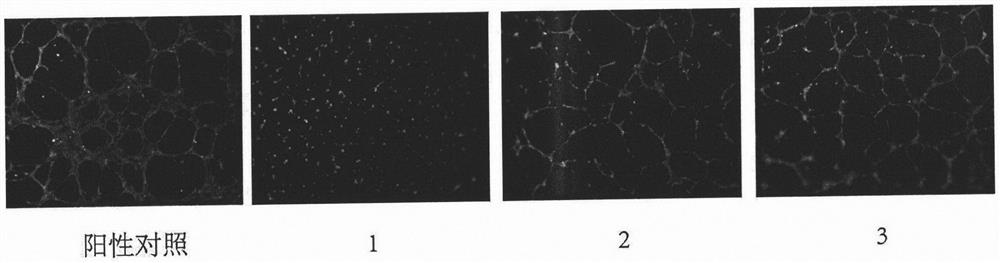
Tripterone 3-site modified derivative and application in preparation of antitumor drugs
A technology of begonia ketone and derivatives, applied to the 3-position modified derivative of begonia ketone and its application in the preparation of antitumor drugs, can solve the problems of unreported pharmacological activities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
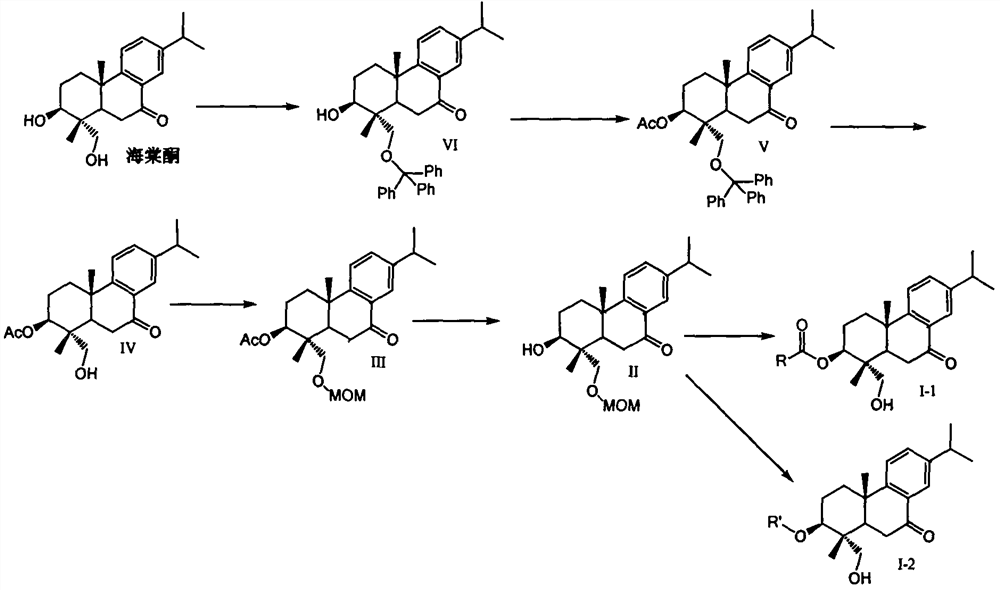
Embodiment 1
[0049] Preparation of compound (IV)
[0050] Weigh 632mg (2.0mmol, 1.0eq) of begonia ketone in a reaction flask, add 4ml of pyridine to dissolve the sample, then add 667mg of triphenylchloromethane (2.4mmol, 1.2eq) and 293mg of dimethylaminopyridine (DMAP , 2.4mmol, 1.2eq). The reaction solution was stirred at room temperature overnight, and TLC detected that the reaction was complete. A large amount of pyridine was concentrated under reduced pressure, a large amount of dichloromethane was added to the residue for dilution, and the organic phase was washed successively with an equal volume of 10% citric acid water, saturated aqueous sodium bicarbonate solution, and saturated brine. The organic phase was dried and concentrated to obtain the crude compound (VI).
[0051] The crude compound (VI) was dissolved in 4ml of pyridine, and then 245mg of acetic anhydride (2.4mmol, 1.2eq) and 293mg of dimethylaminopyridine (DMAP, 2.4mmol, 1.2eq) were added to the reaction solution. The...
Embodiment 2
[0055] Preparation of compound (II)
[0056] Weigh 591mg of compound (IV) (1.65mmol, 1.0eq) in a reaction flask, add 5ml of anhydrous DMF to dissolve it, add 43.6mg of sodium hydride (1.82mmol, 1.1eq) to the reaction system and stir at room temperature for half After 1 hour, 147 mg of methoxymethyl chloride (1.82 mmol, 1.1 eq, MOMCl) was added to the reaction solution, and the reaction solution was stirred at room temperature, and the reaction was monitored by TLC to complete. Dichloromethane was added to the reaction solution to dilute the reaction solution, and the organic phase was washed successively with an equal volume of 10% citric acid water, saturated aqueous sodium bicarbonate solution and saturated brine. The organic phase was dried and concentrated to obtain the crude compound III.
[0057] The crude compound (III) was dissolved in tetrahydrofuran, 4N sodium hydroxide solution was added to the reaction solution, and stirred overnight at room temperature. TLC moni...
Embodiment 3
[0060] Preparation of compound (I-1)
[0061] Weigh 18 mg of compound (II) (1.0 equivalent) in the reaction flask, add 0.5 ml of methylene chloride in the reaction flask, add 1.1 equivalent of condensing agent EDCI, 1.1 equivalent of DMAP and 1.1 equivalent of carboxylic acid (RCOOH) under stirring at room temperature, and react The reaction was continued overnight at room temperature. TLC monitored the completion of the reaction. A large amount of dichloromethane was added to the reaction solution to dilute the reaction solution, and then the organic phase was washed with saturated aqueous sodium bicarbonate and saturated brine. The organic phase was dried, filtered, and concentrated to obtain the crude product. The crude product from the previous step was dissolved in 0.5 ml tetrahydrofuran, then 1N dilute hydrochloric acid was added, and reacted overnight at room temperature. TLC monitored the completion of the reaction. A large amount of dichloromethane was added to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com