Lignan natural product as well as intermediate and preparation method thereof
A technology of natural products and lignans, which can be used in drug combinations, non-central analgesics, antidote and other directions, can solve the problems of mode of action and structure-activity structure-activity relationship, which are rarely reported, and achieve convenient and easy reaction raw materials. Obtain, mild reaction conditions, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] This embodiment provides an intermediate Va of lignan natural products, whose structural formula is as follows:
[0053]
[0054] The intermediate Va of the above-mentioned lignans natural products can be prepared by the following method, comprising the following steps:
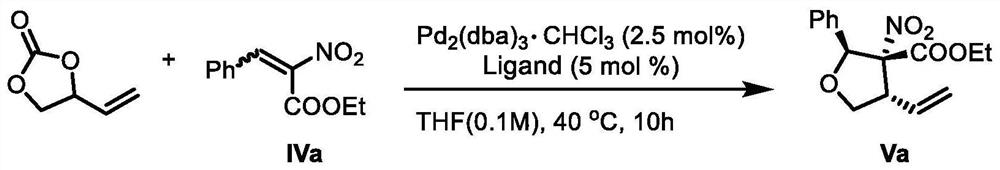
[0055] Pd was added sequentially into the reaction tube 2 (dba) 3 · CHCl 3 (0.025mmol), chiral ligand (0.05mmol), ethylene carbonate (0.6mmol), compound IVa (0.5mmol) and tetrahydrofuran (5.0mL), react at 40°C for 10 hours, the reaction formula is as figure 1 shown. After the solvent was evaporated under reduced pressure, the residue was subjected to column chromatography to obtain lignan natural product intermediate Va (ie intermediate V).
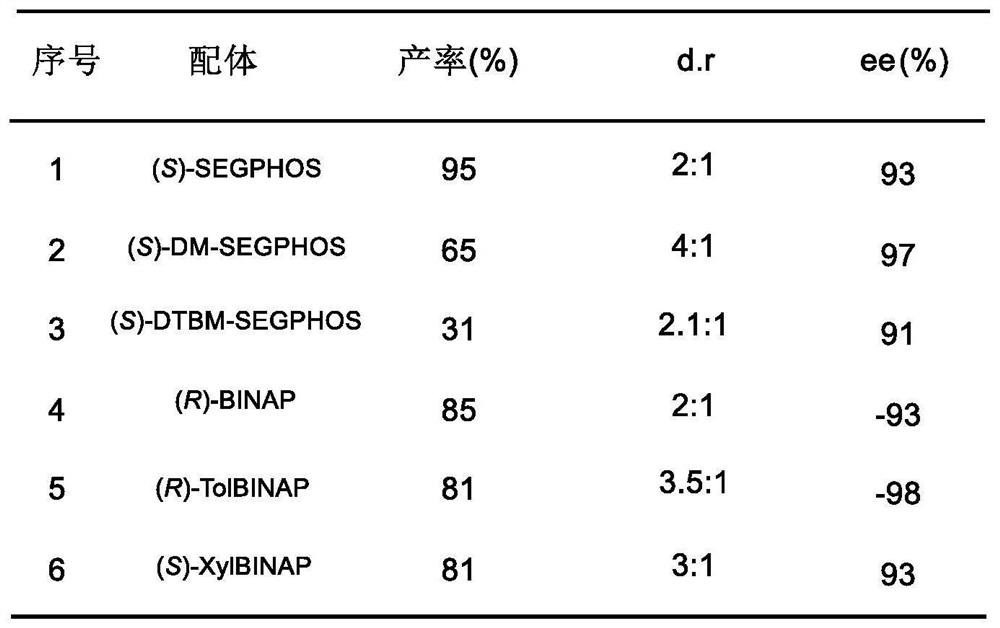
[0056] This example further provides that the ligand selection of the above preparation method is adjusted, and different ligands are used to prepare lignan natural product intermediates, and the rest of the reactants and reaction conditions are the same as...
Embodiment 2
[0058] This embodiment provides an intermediate Va of lignan natural products, whose structural formula is as follows:
[0059]
[0060] The intermediate Va of the above-mentioned lignans natural products can be prepared by the following method, comprising the following steps:
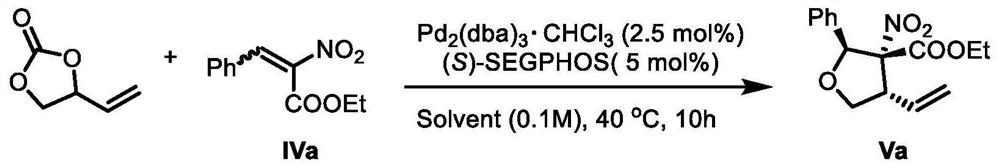
[0061] Add Pd to the reaction tube sequentially 2 (dba) 3 · CHCl 3 (0.025mmol), (S)-SEGPHOS (0.05mmol), ethylene carbonate (1.2mmol), compound IVa (1mmol) and reaction solvent (10mL), react at 40°C for 10 hours, the reaction formula refers to image 3 shown. After the solvent was evaporated under reduced pressure, the residue was subjected to column chromatography to obtain lignan natural product intermediate Va (ie intermediate V).
[0062] This embodiment further provides that the solvent selection of the above-mentioned preparation method is adjusted, and different solvents are used to prepare the lignan natural product intermediate V, and the rest of the reactants and reaction conditions are...
Embodiment 3
[0064] This embodiment provides an intermediate Va of lignan natural products, whose structural formula is as follows:
[0065]
[0066] The above-mentioned a lignan natural product intermediate Va can be prepared by the following method, comprising the following steps:
[0067] Add Pd to the reaction tube sequentially 2 (dba) 3 · CHCl 3 (0.05mmol), (S)-SEGPHOS (0.1mmol), ethylene carbonate (2.4mmol), compound IVa (2mmol) and 1,4-dioxane (20.0mL), reacted for 16 hours, the reaction formula refer to Figure 5 shown. After the solvent was evaporated under reduced pressure, the residue was subjected to column chromatography to obtain lignan natural product intermediate Va (ie intermediate V).
[0068] This embodiment further provides that the reaction temperature of the above-mentioned preparation method is adjusted, and the lignan natural product intermediate V is prepared by using different reaction temperatures. Image 6 It can be seen from the reaction results that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com