A process for nitric acid production
A nitric acid and process technology, which is applied in the production of bulk chemicals, hydrogen/synthesis gas production, chemical industry, etc., can solve the problems of excessive water vapor increase, high temperature, effective steam reforming, multiple The effect of heating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
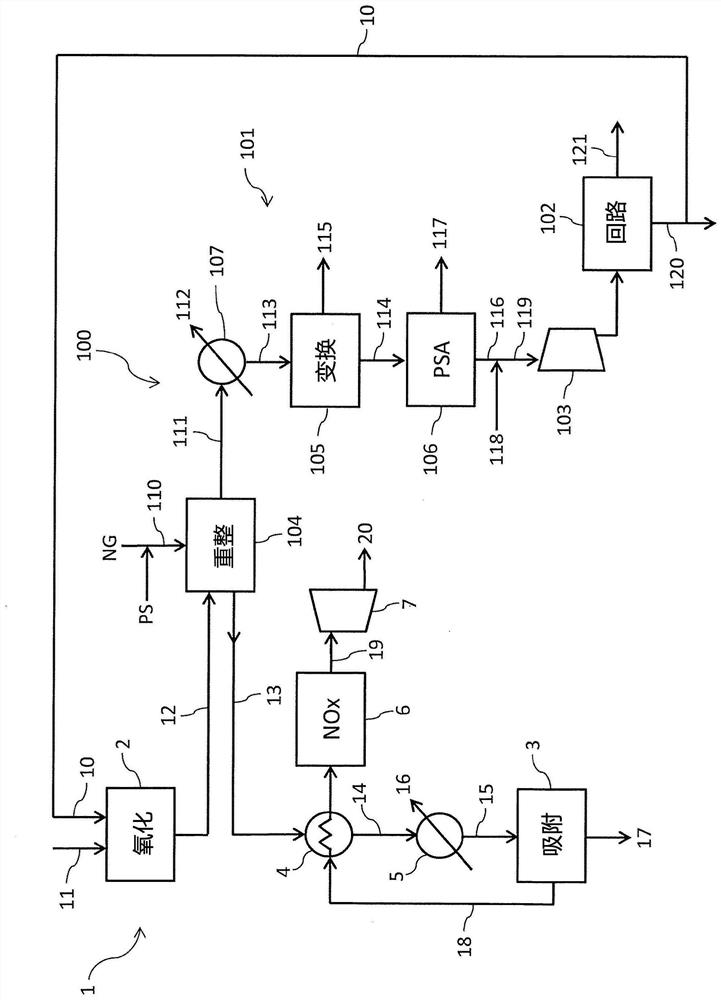
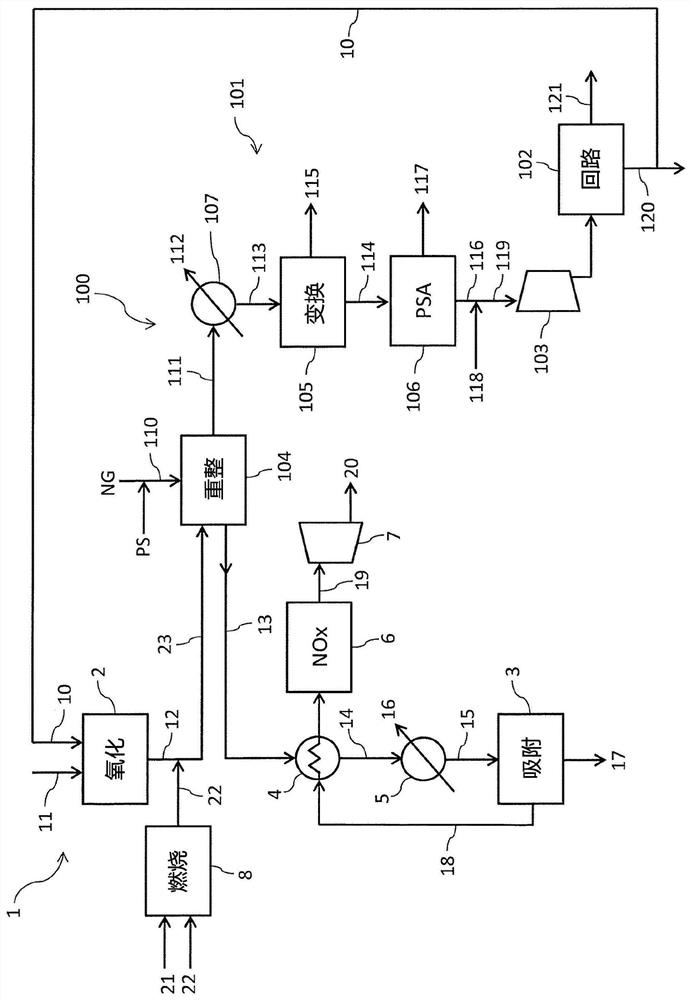
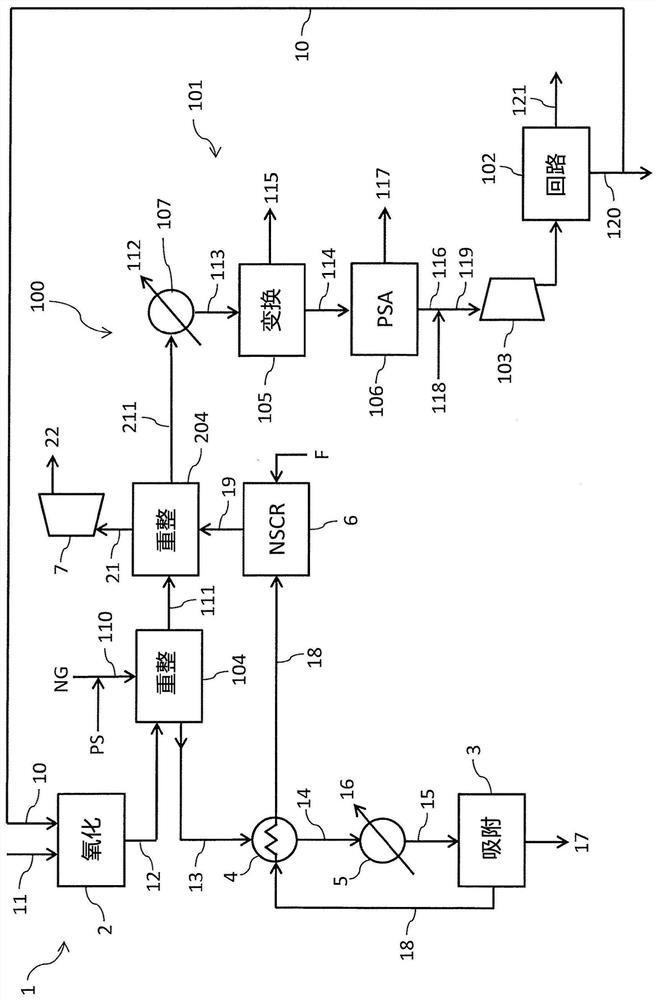
[0105] refer to figure 1 , the advantages of the present invention will be better clarified by the following examples.
[0106] Reference is made to a nitric acid section 1 with a nitric acid production rate of 1500 t / day, where the temperature of the process gas 12 leaving the oxidation reactor 2 is 880°C and is cooled to 500°C. The steam reformer 104 of the ammonia section 100 is fed with a stream 110 having a steam to carbon ratio (S / C) of 3 at a temperature of 450° C. and a pressure of 25 bar. The ammonia consumption of nitric acid is 425 tons / day.
[0107] The nitric acid section 1 provides a steam reformer 104 with a load of 35 Gcal / h and allows obtaining a reformed gas 111 at a temperature of 800°C. Under these conditions, the ammonia production rate was 425 tons / day. From this it can be seen that figure 1 The plant produces enough ammonia to make nitric acid.
[0108] With regard to existing technologies for providing reforming heat by fuel combustion, according t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com