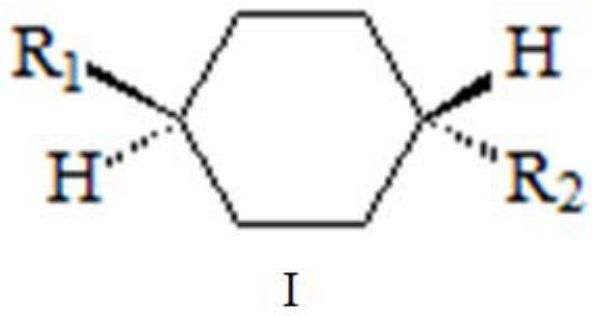
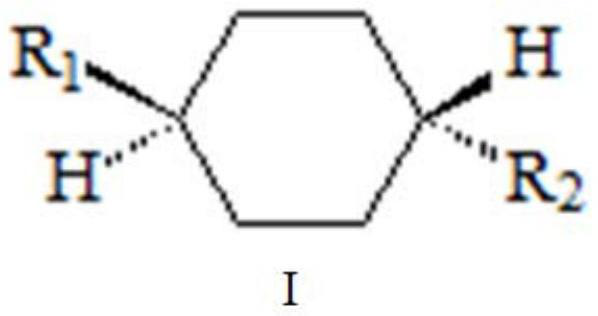
Method for separating and enriching organic matter molecules containing trans-cyclohexyl
A technology for separation and enrichment of organic matter, applied in the field of separation and enrichment of organic matter molecules, which can solve the problems that the configuration transformation method cannot be used and the conversion rate is not high, and achieve the effects of simplified operation, simple process operation and improved recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] From the mixture liquid of trans, trans-4-ethyl-4'-propyl dicyclohexane and cis, trans-4-ethyl-4'-propyl bicyclohexane, separate the enriched trans, trans - 4-Ethyl-4'-propylbicyclohexane.
[0065] Since in Example 1, the two isomers to be separated contain two cyclohexyl groups, and at least one of the cyclohexyl groups is trans, so the efficiency of these two isomers in the separation and enrichment Slightly lower, need to repeat many times.
[0066] The corresponding reaction equation is as follows:
[0067]
[0068]To 118g (0.5mol) of trans, trans-4-ethyl-4'-propyl bicyclohexane (content about 31%) and cis, trans-4-ethyl-4'-propyl bicyclohexane ( content of about 69%), add 300ml of petroleum ether at 20-30°C, and stir well. Another 38g (0.5mol) of thiourea was taken and dissolved in 760g of formamide with stirring at 20-30°C. Then, add the formamide solution of thiourea into the aforementioned 20-30°C cis-trans mixture / petroleum ether solution while stirring;...
Embodiment 2
[0073] From 4'-(2-(trans-4-ethylcyclohexyl)-eth-1-yl)-3,4-difluorobiphenyl, and 4'-(2-(cis-4-ethylcyclohexyl) )-Eth-1-yl)-3,4-difluorobiphenyl mixture, separation and enrichment of 4'-(2-(trans-4-ethylcyclohexyl)-Eth-1-yl)- 3,4-Difluorobiphenyl. The corresponding reaction equation is as follows:
[0074]
[0075] To 32.9g (0.1mol) of 4'-(2-(trans-4-ethylcyclohexyl)-ethyl-1-yl)-3,4-difluorobiphenyl, and 4'-(2-(cis -In the mixture of 4-ethylcyclohexyl)-ethyl-1-yl)-3,4-difluorobiphenyl, add 200ml of toluene at 90~100°C, and stir until all the solids are dissolved. Another 76g (1mol) of thiourea was taken and dissolved in 304g of formamide with stirring at 20-30°C. Then, add the formamide solution of thiourea dropwise to the aforementioned 90-100°C cis-trans mixture / toluene solution while stirring; Large crystals began to appear on the mixture, immediately added 0.3g of methanol, and continued to heat and stir at 1-4°C for 3 hours; then cooled the mixture to -10-5°C and sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com