Application of flavone C-glycoside monomer compound
The technology of a medicine and orientin is applied in the application field of flavonoid carbon glycoside monomer compounds in the preparation of wound healing-promoting medicines, and can solve the problems of high preparation cost, pharmacological activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
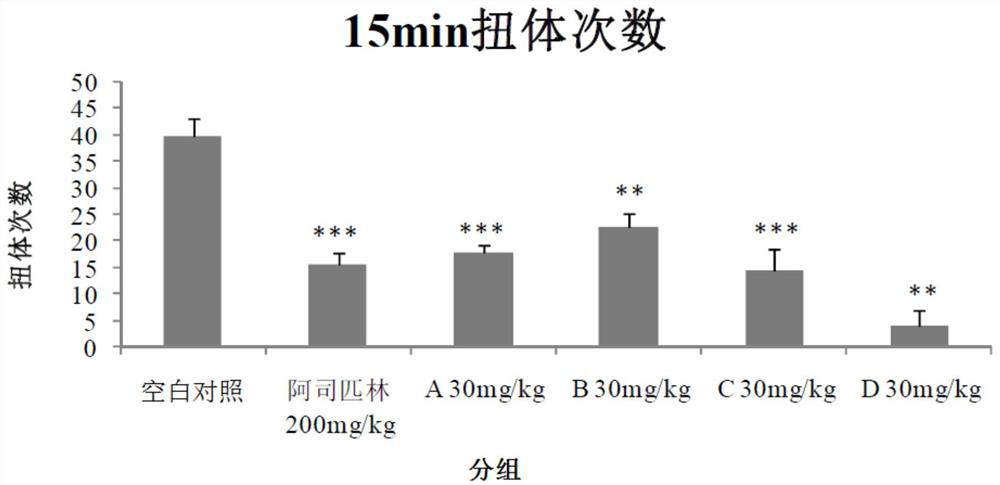
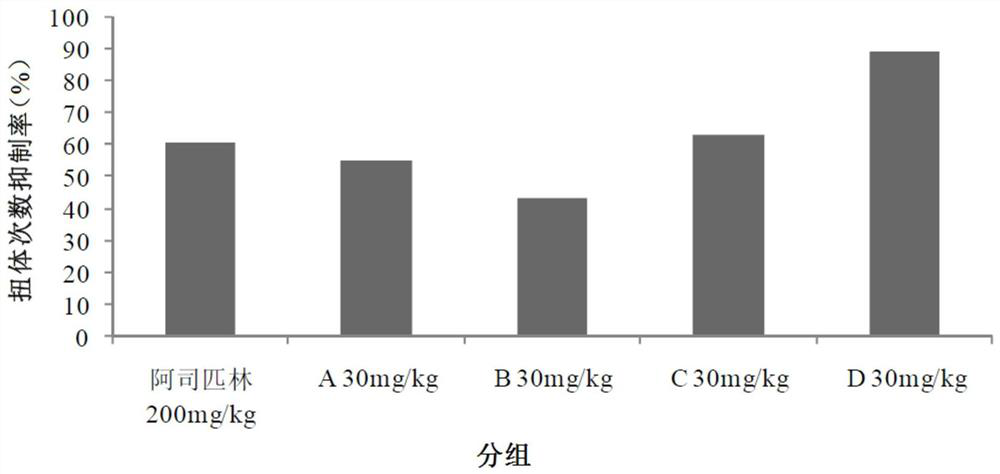
[0040] Example 1 Evaluation of analgesic effect in mouse acetic acid-induced pain model
[0041] Each compound (test substance) is numbered as:
[0042] A: Isovitexin; B: Orientin; C: Vitexin; D: Isorientin
[0043] Blank control (Vehicle): 2g / L physiological saline;
[0044] Positive drug: aspirin enteric-coated tablets.
[0045] After the adaptation period, 60 ICR mice were randomly divided into 6 groups, 10 in each group, and the analgesic experiment was carried out in the mouse acetic acid-induced pain model. The results are shown in Table 1.
[0046] Table 1. Embodiments of the acetic acid-induced pain model in mice
[0047]
[0048] According to Table 1, each group was given blank control or test substances A, B, C and D by intraperitoneal injection, and positive drugs were given by oral gavage. One hour after the administration, the animals were intraperitoneally injected with 0.6% acetic acid solution according to the administration volume of 10 mL / kg. Record t...
Embodiment 2
[0063] Example 2 Analgesic Effect Evaluation of Formalin Plantar Pain Model
[0064] Each compound (test substance) is numbered as:
[0065] A: Isovitexin; B: Orientin; C: Vitexin; D: Isorientin
[0066] Blank control (Vehicle): 2g / L physiological saline;
[0067] Positive drug: rotundine.
[0068] After the adaptation period, 60 mice were randomly divided into 6 groups, and 10 mice in each group were subjected to the following experiments. See Table 4.
[0069] Table 4. Analgesic implementation plan of formalin plantar pain model
[0070]
[0071] According to Table 4, each group was given blank control or test substances A, B, C and D by intraperitoneal injection, and positive drugs were given by oral gavage. One hour later, 10 μL of 1.5% formaldehyde solution was injected into the plantar of the animals. After the injection of formaldehyde solution, the animals were scored according to the following criteria within four time periods of 0-10 minutes, 10-30 minutes, ...
Embodiment 3
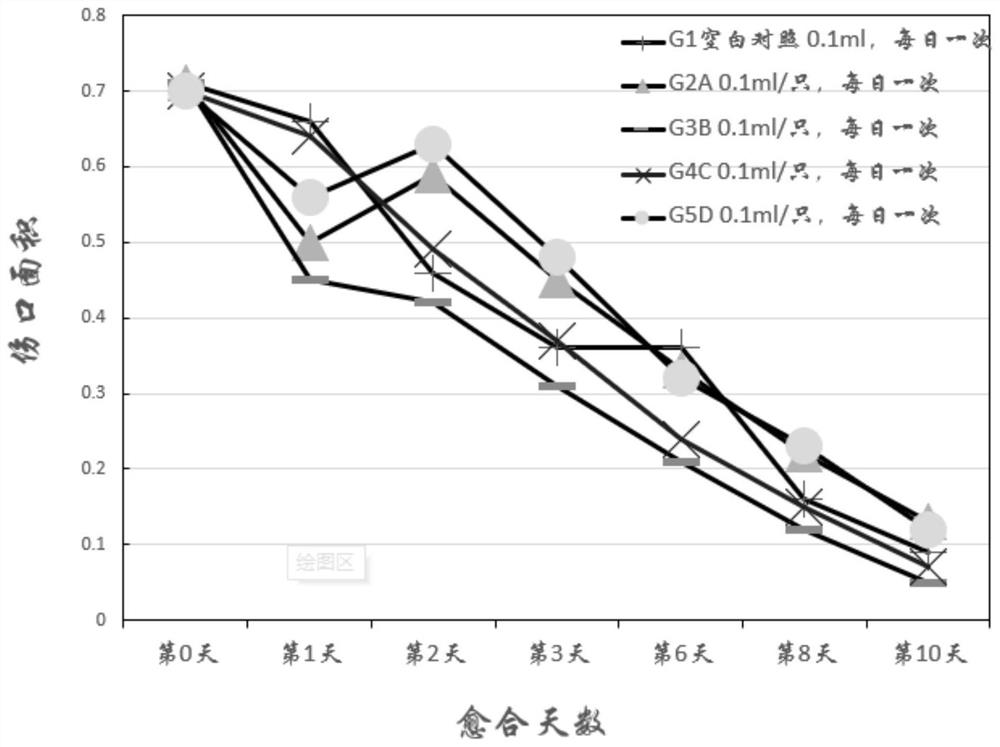
[0095] Embodiment 3 Evaluation of rat incision wound healing effect
[0096] Each compound (test substance) is numbered as:
[0097] A: Isovitexin; B: Orientin; C: Vitexin; D: Isorientin
[0098] Blank control (Vehicle): 2g / L physiological saline.
[0099] After the adaptation period, 70 rats were anesthetized by 10% chloral hydrate (300mg / kg, intraperitoneal injection), and the backs were shaved and disinfected. A wound of 2 cm in length was cut with a scalpel on the back of the rats, and photographed and recorded. Tracing the wound, using Image-Pro The software calculates the wound area. According to the wound area, 50 animals were randomly divided into 5 groups with 10 animals in each group. The results are listed in Table 6.
[0100] Table 6, rat incision wound healing evaluation embodiment
[0101]
[0102] According to Table 6, each group was given experimental rats blank control or test substances A, B, C, and D, respectively, and took pictures and calculated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com