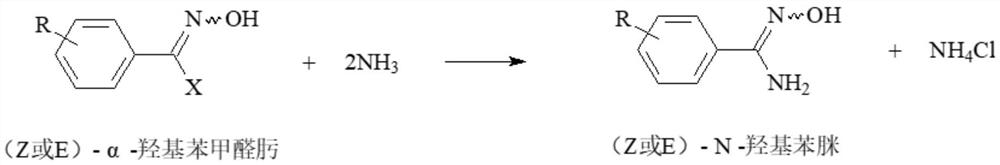
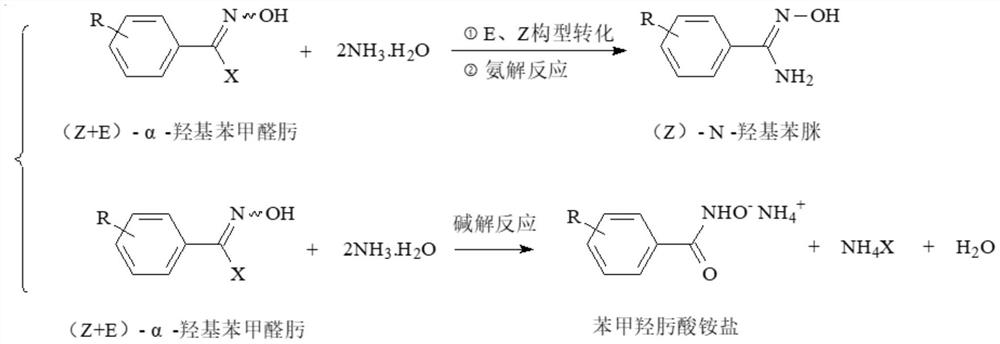
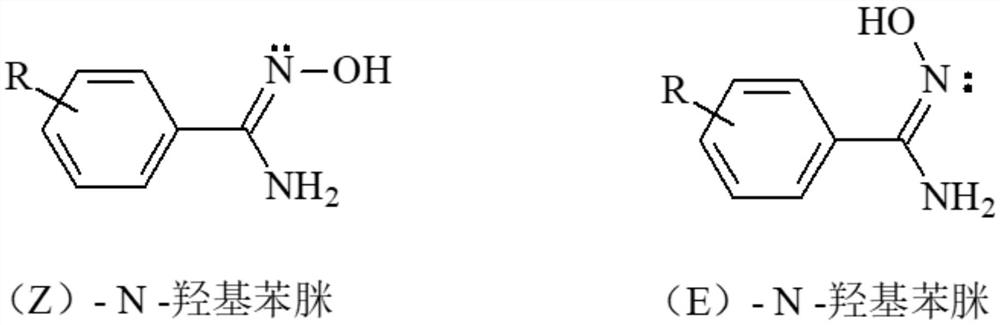Method for preparing (Z)-N-hydroxyphenylamidine by ammonolysis method
A technology of hydroxybenzamidine and solution method, which is applied in the field of preparation of -N-hydroxybenzamidine by ammonolysis method, can solve the problems of difficult separation, low yield, difficult separation, etc., and achieve the effect of improving the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (Z)-N-hydroxyl-2,3-difluoro-5-trifluoromethylphenylamidine was prepared by ammonolysis, and the reaction equation was as follows:
[0063]
[0064] Take 51.9g (0.2mol) of α-chloro-2,3-difluoro-5-trifluoromethylbenzaldehyde oxime (E, Z configuration mixture) and dissolve it in 250ml of absolute ethanol, then add 1.66g (0.006mol) of the anhydrous potassium salt of 2,3-difluoro-5-trifluoromethylbenzohydroxamic acid, keep warm at 0-5°C and stir for 24 hours, then heat up to 20-30°C, and stir in batches A total of 15.8 g (0.2 mol) of ammonium bicarbonate was added; after the addition was complete, the reaction was still stirred at 20-30° C. for 24 hours. Concentrate under reduced pressure with a rotary evaporator to remove most of the ethanol, add 200 g of water and 200 g of chloroform to the concentrated residue, and stir at 30-40° C. until most of the solids are dissolved. Filtrate while it is hot, and the obtained filter cake is the potassium salt of 2,3-difluoro-5-tr...
Embodiment 2
[0074] Adopt ammonolysis method to prepare (Z)-N-hydroxyl-4-trans-(4-ethyl)cyclohexylphenylamidine, and the reaction equation is as follows:
[0075]
[0076] In a 1000ml autoclave, mix 62.0g (0.2mol) of α-bromo-4-trans-(4-ethyl)cyclohexylbenzaldehyde oxime (E, Z configuration mixture) with 200ml of anhydrous isopropyl Alcohol and 100ml of toluene were mixed, then 3.18g (0.02mol) of anhydrous sodium salt of benzohydroxamic acid was added, and the mixture was kept at 55-60°C and stirred for 1 hour. Then add an ammonia gas ethanol solution containing a total of 6.8 g of ammonia (0.4 mol), seal the autoclave, heat up to 90-100° C. under stirring and react for another hour. Then cool down to below 40°C, take out the reaction mixture, concentrate under reduced pressure with a rotary evaporator to remove most of the isopropanol, add 200g of water and 200ml of toluene to the concentrated residue, and stir at 30-40°C until most of the solids dissolve. Filtration, the obtained fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com