Synthesis and application of a fluorescent sensor capable of single and selective recognition of l-arginine
A fluorescent sensor, arginine technology, applied in fluorescence/phosphorescence, instruments, luminescent materials, etc., can solve the problem of slow generation speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
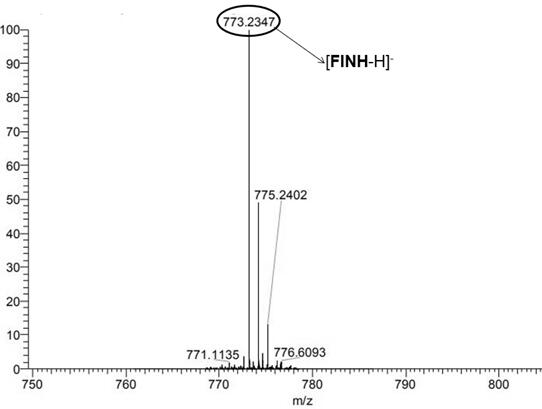
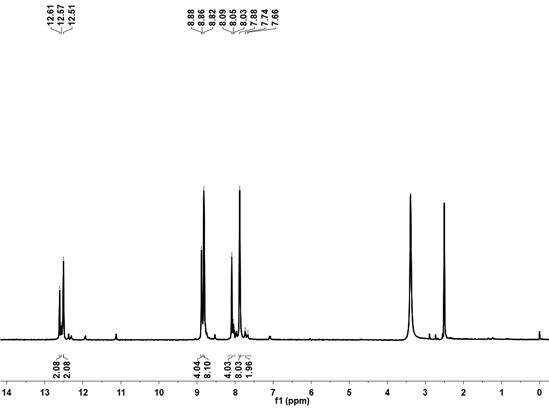
[0031] Example 1, the fluorescent sensor FINH
[0032](1) Synthesis of 4,4 ́-bisorcinol derivatives: weighing 0.9g (50mmol) 4,4 ́-bisphenol, 17g of six methyltetraamines (500mmol) was added to 10mL trifluoroacetic acid, reacted at 140 °C for 72 h; after the reaction, dilute hydrochloric acid was added, stirred and filtered, the product was an orange solid, recrystallized with dimethyl sulfoxide, 7.45g4,4 ́- bisclicdiol derivative was obtained, the yield was 52%;
[0033] (2) Synthesis of fluorescence sensor FINH: weigh 0.82g (4mmol) isoniazid and 0.29g (1mmol) 4,4 ́-biphenyldiol derivatives were added to 20mL of absolute ethanol, reacted at 80 °C for 24h, after the end of the reaction precipitated yellow solid, filtered, washed with hot ethanol, the resulting product is the fluorescent sensor FINH. The yield was 35 per cent.
Embodiment 2
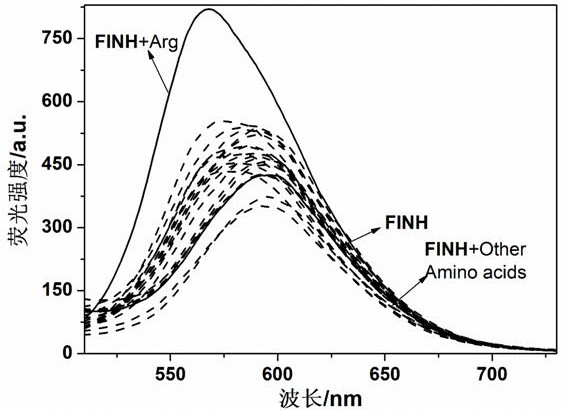
[0034] Example 2, the fluorescent sensor FINH identification L- arginine
[0035] Pipette the DMSO-H of the 2 mL fluorescent sensor FINH 2 O solution (C FINH =8×10 -5 M,V DMSO : V 水 = 3:2) In a series of colorimetric tubes, L-glycine, L-alanine, L-valine, L-leucine, L-isoleucine, L-methionine (methionine), L-proline, L-tryptophan, L-serine, L-tyrosine, L-cysteine, L-phenylalanine, L-asparagine, L-glutamine, L-threonine, L-aspartic acid, L-glutamic acid, L-glutamic acid, L-lysine, L-arginine and L-histidine aqueous solution (C=0.1M), DMSO-H for sensor molecules 2 The fluorescence of the O solution is significantly enhanced, indicating that the L-arginine solution is added; if the fluorescence intensity of the sensor molecule does not change, it means that the addition is not L-arginine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com