A kind of cardiac glycoside compound and its synthesis method and application
A synthesis method and compound technology, applied in the field of medicine, can solve the problems of adverse reactions of normal cells and difficult to guarantee safety, and achieve the effect of strong anti-tumor effect in vivo, good safety and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment provides a cardiac glycoside compound 19-dihydrocalotoxin, which is a compound represented by formula (I) or a pharmaceutically acceptable salt thereof;
[0032]
[0033] The synthetic method of this cardiac glycoside compound (19-dihydrocalotoxin) is:
[0034] Calotoxin (215 mg, 0.39 mmol) was dissolved in methanol (20 mL) precooled in an ice bath, solid sodium borohydride (52 mg, 1.3 mmol) was added in portions, and the above solution was stirred at room temperature for 2 hours. After detecting the complete disappearance of the reaction raw materials by TLC (chromatographic chromatography), add saturated NH 4 Aqueous Cl (6.5 mL) was used to quench the reaction. After methanol was recovered under reduced pressure, the aqueous solution was extracted with ethyl acetate (EtOAc), and after the organic solvents were combined, they were washed with brine (5 mL) successively, anhydrous Na 2 SO 4 Dry, filter, and recover the organic solvent under reduced ...
Embodiment 2
[0038] Cytotoxicity of cardiac glycoside compound 19-dihydrocalotoxin on tumor cell line T47D
[0039] Human breast ductal carcinoma cells T47D were cultured in DMEM complete medium, and the growth inhibitory effect of the cardiac glycoside compound 19-dihydrocalotoxin in Example 1 on T47D tumor cells was detected by MTT assay. The specific experimental procedure was as follows:
[0040] Cells in the logarithmic growth phase were planted in a 96-well plate, 5×10 per well 3 After the cells adhere to the wall, treat the cells with a series of different concentrations of drug-containing medium for 24 hours, set up three duplicate wells, and set up blank control wells and zero-adjustment wells at the same time, and then add 10 μL of 5 mg / mL to each well. After further incubation for 4 hours, the culture medium was removed, and 100 μL DMSO was added to each well to dissolve the formed formazan, and then the absorbance value was measured at a wavelength of 570 nm. The cell growth i...
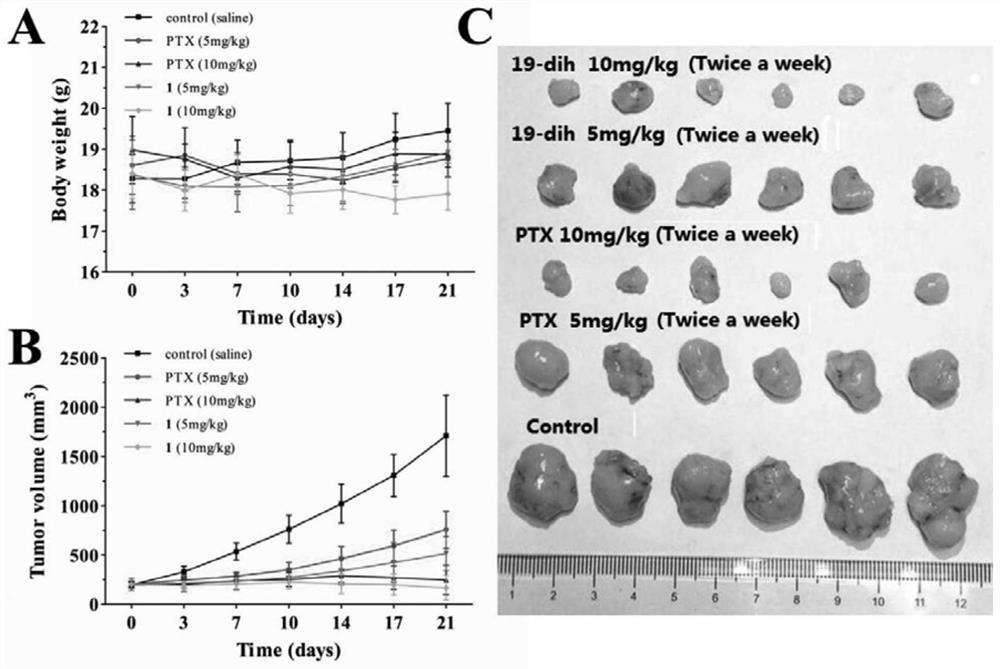
Embodiment 3
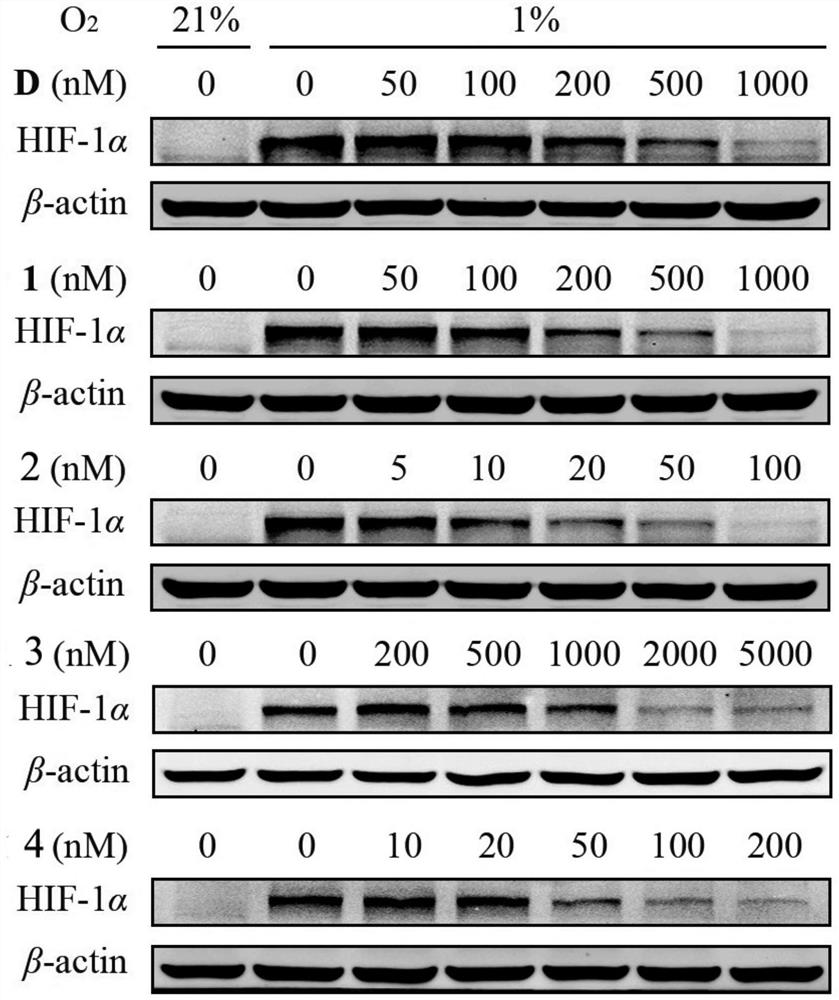
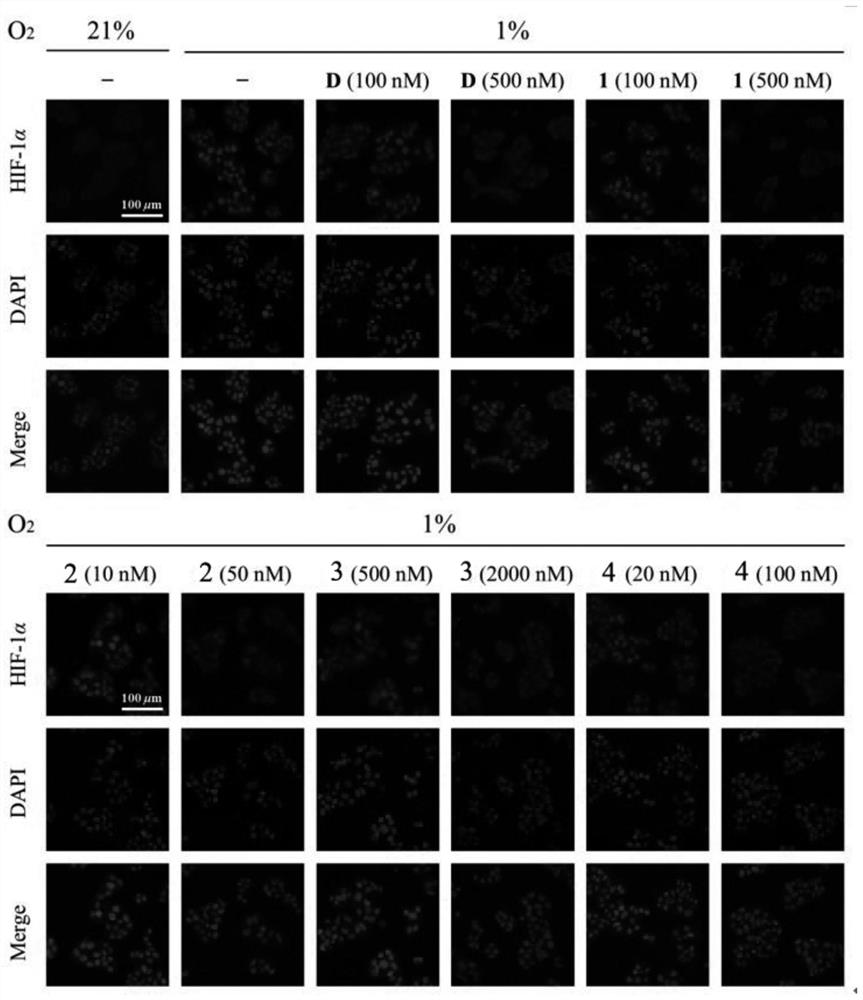
[0047] Inhibitory Effect of Cardiac Glycoside Compound 19-dihydrocalotoxin on HIF-1α Protein Expression (Western Blotting Experiment)
[0048] In order to confirm whether the inhibitory effect of cardiac glycosides (19-dihydrocalotoxin) on HIF-1 transcriptional activity in Example 1 is caused by its inhibition of HIF-1 activity or reduction of HIF-1 protein expression, western blot experiments were used to detect representative Effects of cardiac glycosides on HIF-1α protein expression in tumor cells under hypoxic conditions. The T47D cells were planted in 6-well plates, and the cells were treated with different concentrations of cardiac glycoside-containing medium after adherence, and the cells were placed under hypoxic conditions for 24 hours, and normoxic conditions (oxygen concentration 21%) and Blank control wells under hypoxic conditions (oxygen concentration 1%). After the cell treatment, the cell protein was extracted, electrophoresis experiment and immunoblotting exp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com