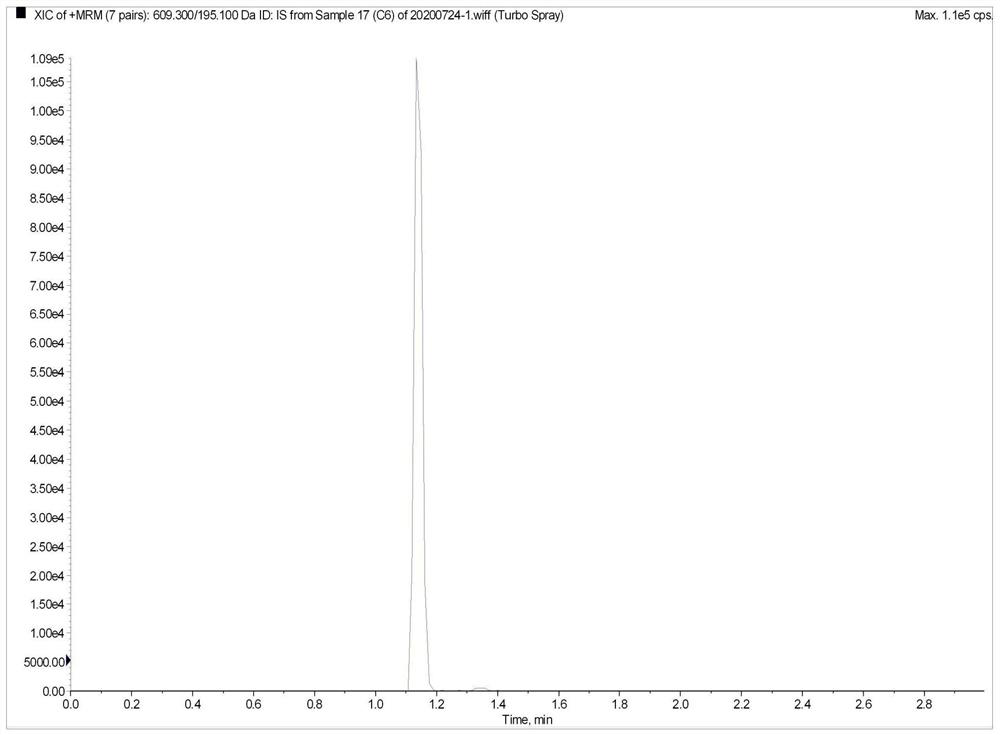
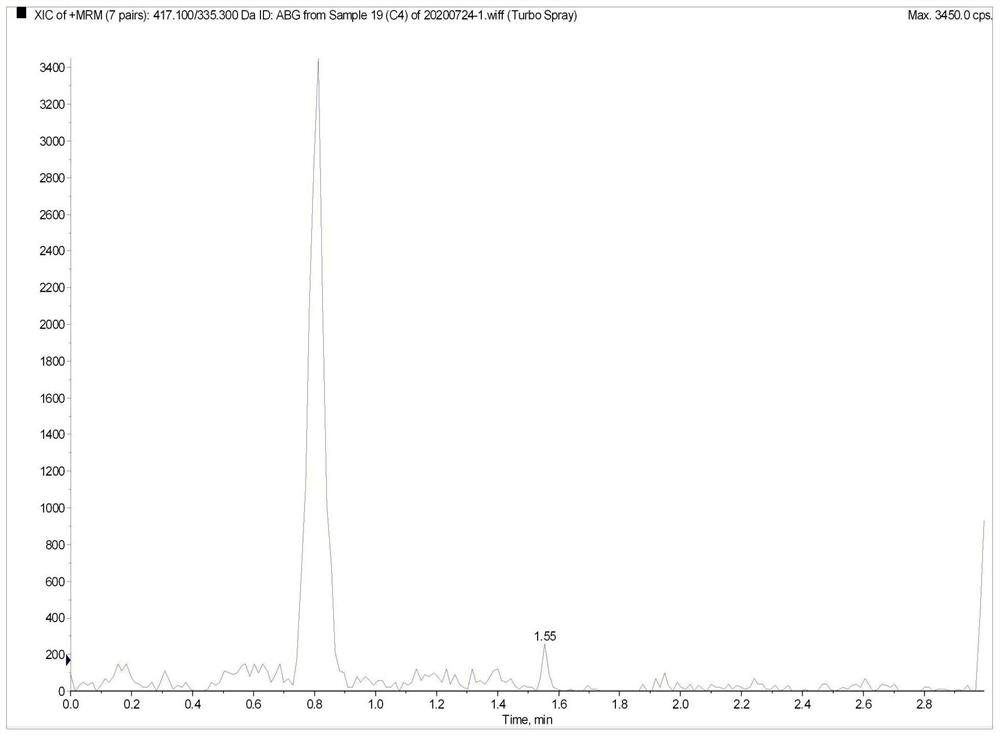
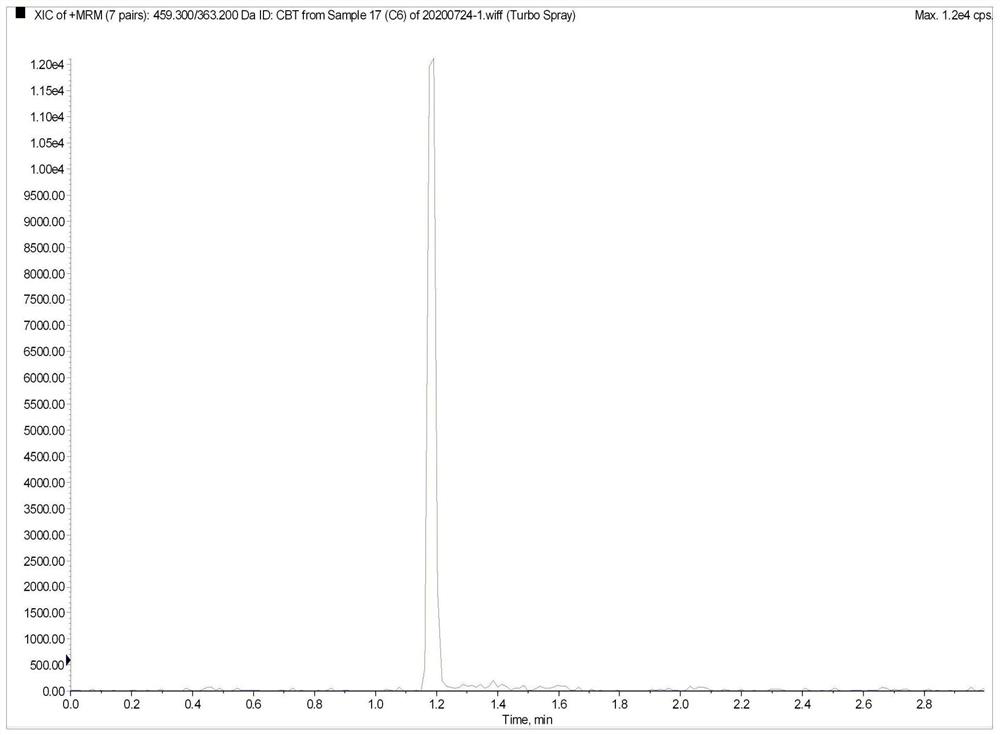
Method for the determination of plasma or tissue concentrations of six bufastenolide components in bufa skin-containing medicinal preparations
A technology of bufastenolactone and detection method, which is applied in the direction of material separation, measuring device, and analysis of materials, can solve the problem of unloaded toad skin, etc., and achieve simplified sample pretreatment process, simple operation, and high resolution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] (1) Preparation of stock solution:
[0066] Take the standard products lipobufaxin, bufalin, cinobufogen, cinobufalin, celinobufagin, xenobufalin, and dissolve them in (50±5)% acetonitrile aqueous solution respectively to prepare the standard product stock solution; mix the standard stock solution to obtain a mixed standard stock solution;
[0067] Dissolve reserpine in (50±5)% acetonitrile aqueous solution to prepare reserpine stock solution;
[0068] (2) Preparation of working solution:
[0069] Dilute the mixed standard stock solution with (50±5)% acetonitrile aqueous solution respectively to prepare a mixed standard curve sample working solution and a mixed quality control sample working solution;
[0070] Dilute the reserpine stock solution with (50±5)% acetonitrile aqueous solution to prepare an internal standard working solution;
[0071] (3) Preparation of sample solution to be tested:
[0072] Preparation of the plasma sample solution to be tested: take the...
Embodiment 1
[0133] (1) Preparation of solution
[0134] Preparation of stock solutions:
[0135] Weigh an appropriate amount of the standard products of the main components of bufaxin, bufalin, cinobufogen, cinobufogen, celinobufosin, and xenobufalin, respectively, and dissolve them in an appropriate amount of 50% acetonitrile. In aqueous solution, standard stock solutions with concentrations of 1.00 mg / mL were prepared. Three standard stock solutions with a concentration of 1.00 mg / mL were mixed in equal volumes, and an appropriate amount of 50% acetonitrile aqueous solution was added to prepare a mixed standard stock solution with the concentration of six bufastenolide components of 0.100 mg / mL.
[0136] Take an appropriate amount of reserpine standard and dissolve it in a 50% acetonitrile aqueous solution to prepare a reserpine stock solution with a concentration of 1.00 mg / mL.
[0137] Preparation of standard curve working solutions:
[0138] Dilute the stock solutions of the six b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision energy | aaaaa | aaaaa |
| collision energy | aaaaa | aaaaa |
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com