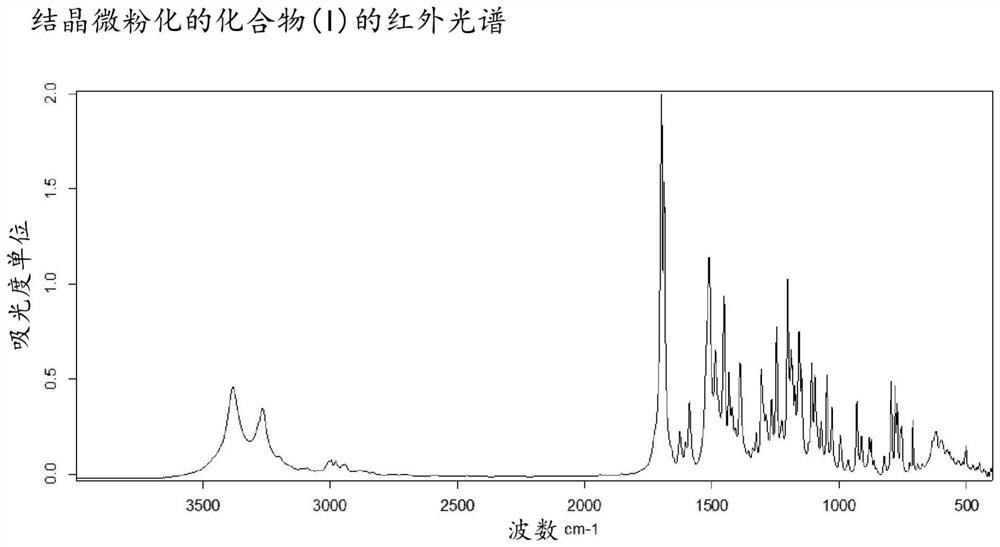
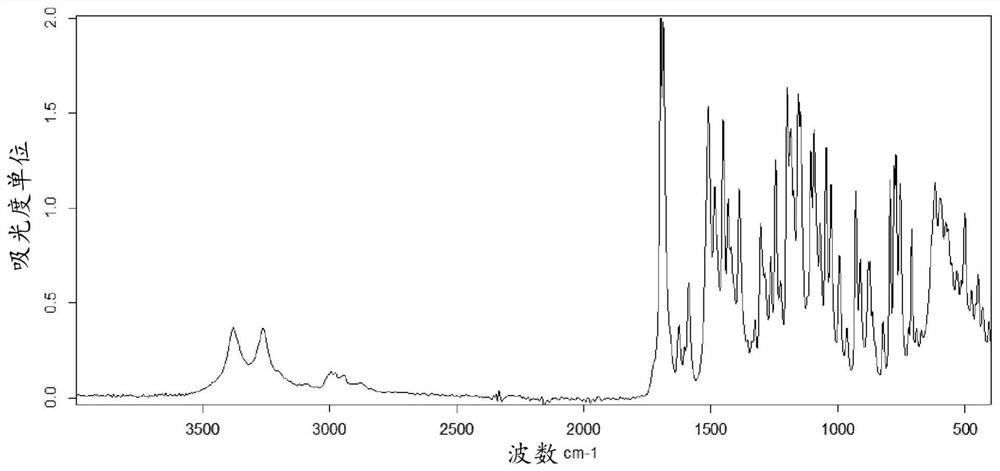
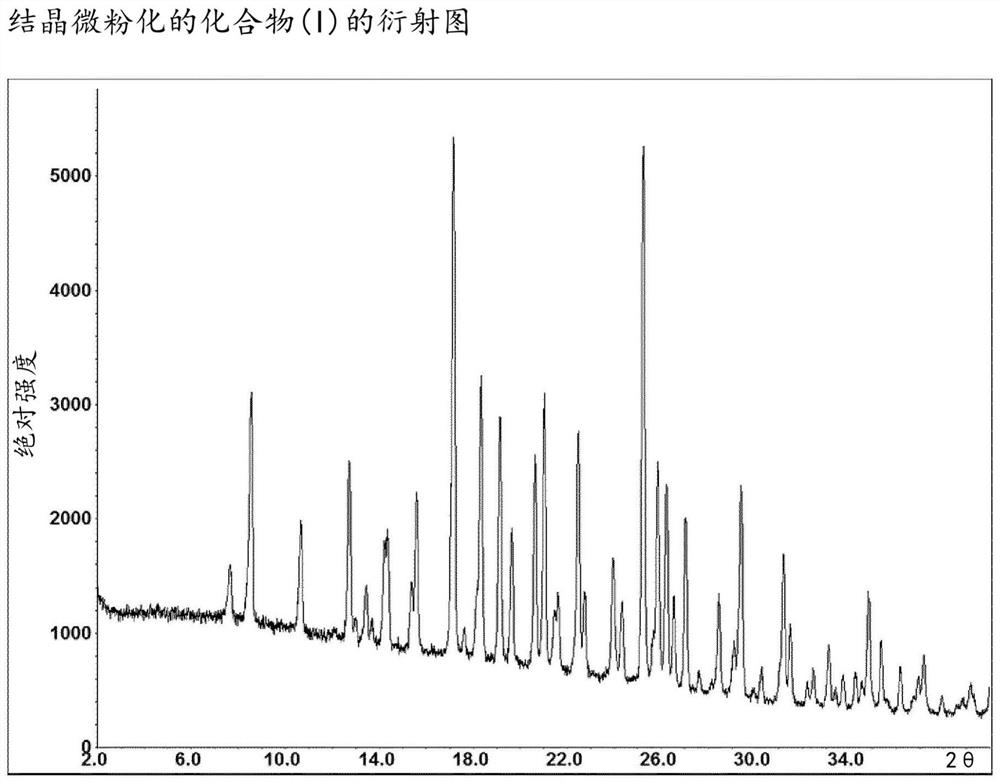
Method for the preparation of a 2,4,5-trisubstituted 1,2,4-triazolone
A compound, suitable technology, applied in the direction of organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as low conversion rate, limited availability, large-scale composition problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0591] 2-Tetrahydropyran-2-yloxyacetylhydrazide (A1)
[0592]
[0593] First, 450 g (3.40 mol) of butyl glycolate (xxx1) were added to 2250 ml of dichloromethane. 5.5 g (0.03 mol) of 4-toluenesulfonic acid monohydrate were added at 20°C. 3,4-Dihydro-2H-pyran (309 g, 3.68 mol) (xxx2) was added over 30 minutes at an internal temperature below 35°C. The mixture was then cooled to 22°C and stirred for a further 16 hours.
[0594] 1125 ml of saturated aqueous sodium bicarbonate solution were added, the organic phase was separated and first evaporated at 50°C bath temperature / 3 mbar reduced pressure, yielding 751 g of a dark orange oil. Further distillation to remove impurities (80°C bath temperature / 0.25 mbar) afforded 659 g of (A1 ) as a dark orange oily distillation residue, 89% yield.
[0595] This crude product was converted directly in the next stage.
[0596] 1 H NMR (400MHz, DMSO-d 6 )δppm 0.89 (t, J=7.40Hz, 3H) 1.33 (dq, J=14.93, 7.41Hz, 2H) 1.39-1.79 (m, 10H) 3.38...
Embodiment 2
[0599] 4-Ethyl-3-(tetrahydropyran-2-yloxymethyl)-1H-1,2,4-triazol-5-one (A4)
[0600]
[0601] Phase 1:
[0602]
[0603] At 20 °C, 375 g (1.734 mol) of intermediate (A1) were charged to the reactor, 104.2 g (2.081 mol) of hydrazine hydrate were added, and the mixture was heated to an internal temperature of 57 °C for 3 hours (slight reflux) . The mixture was then cooled to 22°C.
[0604] This crude intermediate (A2) was used directly in the next stage.
[0605] Intermediate (A2) was previously described in DE 2156472 (Ciba Geigy 1971).
[0606] Phase 2:
[0607]
[0608] Water (906ml) was added to intermediate (A2), and the mixture was cooled to 10°C. Ethyl isocyanate (172.6 g, 2.428 mol) was added over 50 minutes at an internal temperature of 10-15°C. The mixture was then warmed to 20°C over 30 minutes.
[0609] The crude reaction mixture of intermediate (A3) was used directly in the next stage.
[0610] Phase 3:
[0611]
[0612] 50% aqueous sodium hydr...
Embodiment 3
[0622] 4-[4-Ethyl-5-oxo-3-(tetrahydropyran-2-yloxymethyl)-1,2,4-triazol-1-yl]-2,5-difluoro -Benzonitrile (A5)
[0623]
[0624] Intermediate (A4) (250 g, 1.10 mol) and 2,4,5-trifluorobenzonitrile (xxx5) (190 g, 1.21 mol) were stirred in acetonitrile (2500 mL), and potassium phosphate (467 g, 2.20 mol) was added . The mixture was stirred at 70-73°C for 20 hours, then cooled to room temperature.
[0625] Water (1200 mL) was added, and the mixture was stirred for 15 minutes. The organic phase was separated, washed with 10% aqueous sodium chloride (1200 mL) and evaporated under reduced pressure at a bath temperature of 40°C to give 414 g of a brown viscous oil.
[0626] The residue was dissolved in absolute ethanol (915 mL) at 40°C, then cooled to 25°C to initiate crystallization. Water (1250 mL) was added and the suspension was stirred at 22°C.
[0627] After stirring for 16 hours, the solid was isolated, washed twice with ethanol / water (3:4v / v, 245mL), and dried under va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size distribution | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com