Preparation method of palladium-based supported hydrogenation catalyst and catalyst thereof
A hydrogenation catalyst and supported technology, which is applied in the field of preparation of palladium-based supported hydrogenation catalysts, can solve problems such as poor catalytic performance, particle aggregation, and inability to accurately control the dispersion of active component palladium, and achieve low manufacturing costs and high efficiency. High hydrogen activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
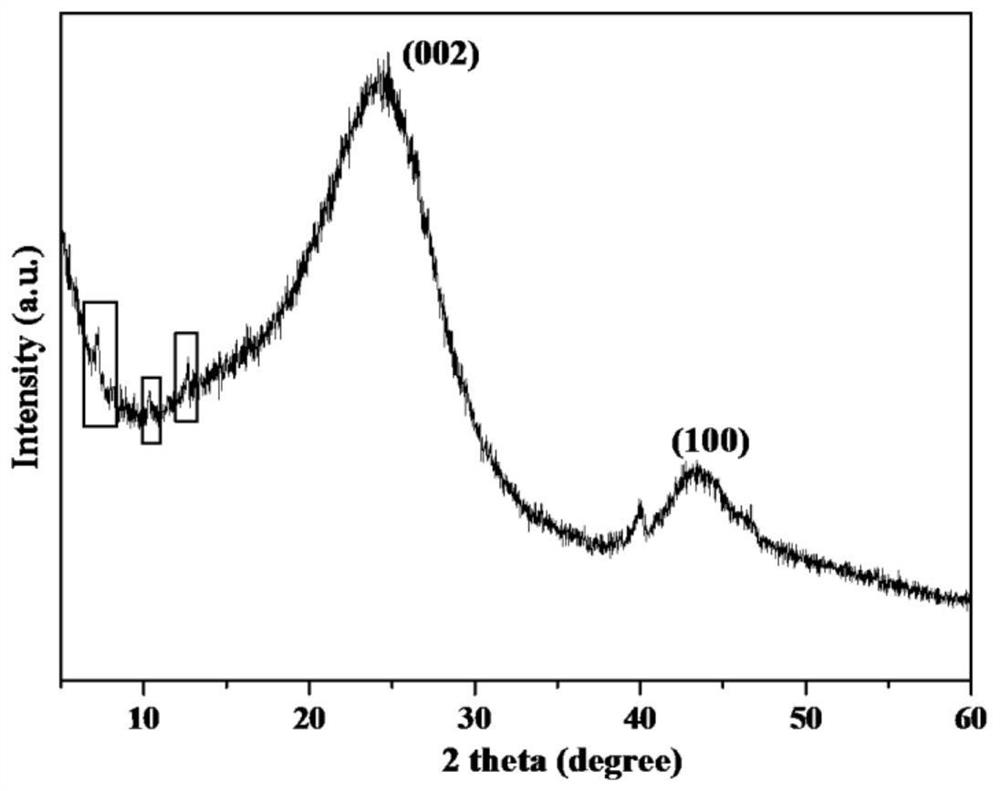
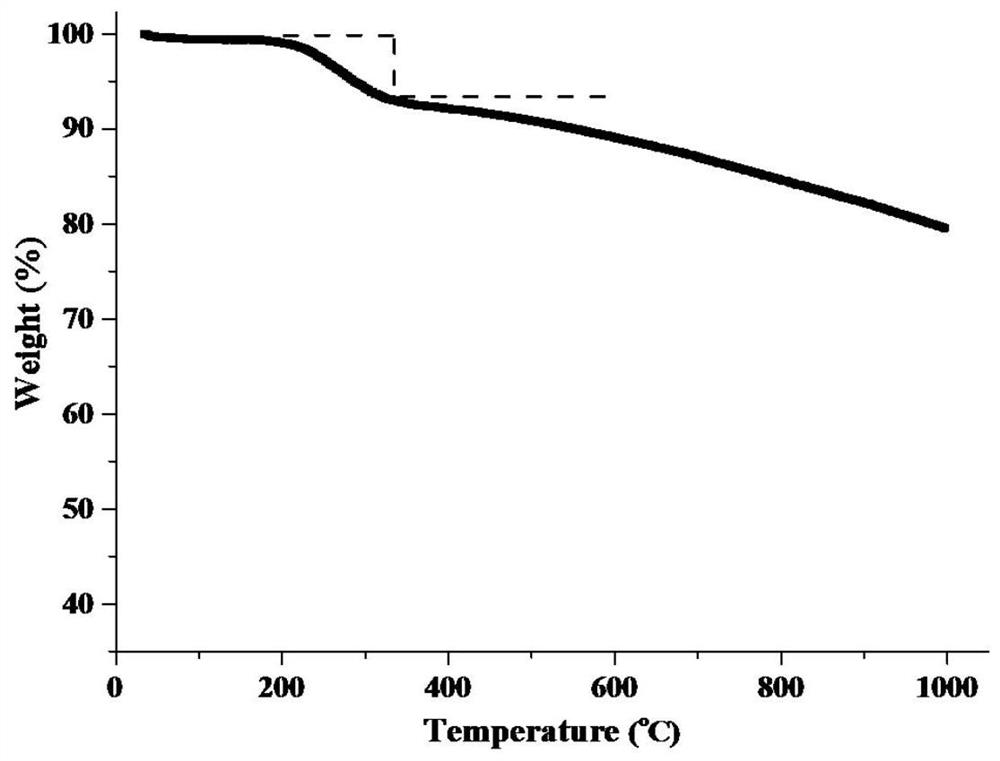
[0029] Step (1), zinc nitrate hexahydrate (0.297g), polyvinylpyrrolidone (0.3g) and sodium chloropalladate solution (0.05mol / L, 3ml) were dissolved in 10ml methanol solution, 4.0g gac was dispersed in 10ml In the methanol solution, join in the above-mentioned salt solution, obtain system I; Step (2), dissolve 2-methylimidazole (0.082g) in the 10ml methanol solution, and join it in the system I; Step (3) , transfer the turbid liquid obtained in step (2) to a 250ml reaction kettle with a polytetrafluoroethylene liner, feed 1.0MPa hydrogen, react at 60°C for 2h, and the product is suction filtered, washed, and dried to obtain a catalyst Precursor; step (4), the precursor sample obtained in step (3) was treated at 900° C. for 3 hours under nitrogen atmosphere to obtain catalyst A. The XRD figure and thermogravimetric curve figure of the catalyst precursor prepared in step (3) are shown in respectively figure 1 and figure 2 .
Embodiment 2
[0031] Step (1), zinc nitrate hexahydrate (0.297g), polyvinylpyrrolidone (0.3g) and sodium chloropalladate solution (0.05mol / L, 3ml) were dissolved in 10ml methanol solution, 4.0g gac was dispersed in 10ml In the methanol solution, join in the above-mentioned salt solution, obtain system I; Step (2), dissolve 2-methylimidazole (0.164g) in the 20ml methanol solution, and join it in the system I; Step (3) , transfer the turbid liquid obtained in step (2) to a 250ml reaction kettle with a polytetrafluoroethylene liner, feed 1.0MPa hydrogen, react at 60°C for 2h, and the product is suction filtered, washed, and dried to obtain a catalyst precursor body; step (4), the sample obtained in step (3) was treated at 900° C. for 3 h under a nitrogen atmosphere to obtain catalyst B.
Embodiment 3
[0033] Step (1), zinc nitrate hexahydrate (0.297g), polyvinylpyrrolidone (0.3g) and sodium chloropalladate solution (0.05mol / L, 3ml) were dissolved in 10ml methanol solution, 4.0g gac was dispersed in 10ml In the methanol solution, join in the above-mentioned salt solution, obtain system I; Step (2), dissolve 2-methylimidazole (0.246g) in the 30ml methanol solution, and join it in the system I; Step (3) , transfer the turbid liquid obtained in step (2) to a 250ml reaction kettle with a polytetrafluoroethylene liner, feed 1.0MPa hydrogen, react at 60°C for 2h, and the product is suction filtered, washed, and dried to obtain a catalyst precursor body; step (4), the sample obtained in step (3) was treated at 900° C. for 3 h under a nitrogen atmosphere to obtain catalyst C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com