Method for preparing N<epsilon>-lysine-based surfactant by using microreactor
A surfactant and lysine-based technology, applied in the field of enzyme-catalyzed amidation of lysine, can solve the problems of low selectivity, long reaction time, low yield, etc., achieve simple preparation process and improve selectivity , Optimizing the effect of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
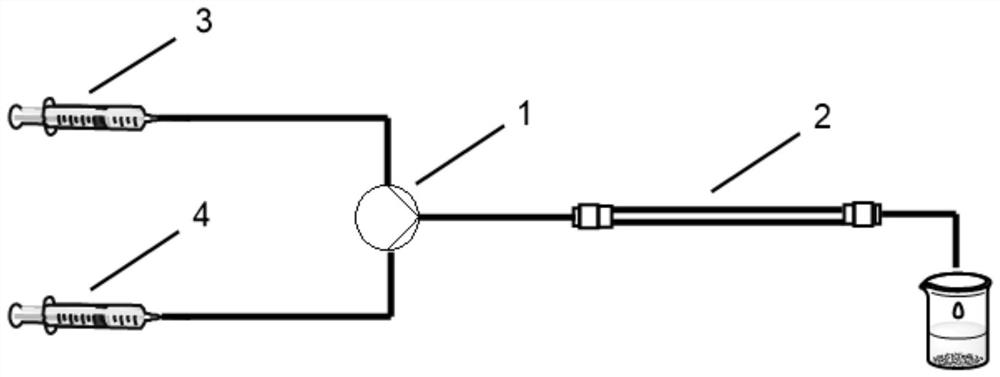
[0025] Fill 0.495 g of immobilized enzyme Novozyme 435 with a particle size of 0.3 to 0.9 mm into an immobilized enzyme microreactor with an inner diameter of 3.8 mm and a length of 150 mm (retention volume is 1.2 mL), connect the device, and use n-hexane solvent Flush the pipes. The concentration is 80mmol·L -1 The lysine is dissolved in water to obtain the mixed solution A, which is transferred to the device 3, and the concentration is 20mmol·L -1 octanoic acid dissolved in n-hexane to obtain mixed solution B Move it into device 4, adjust the flow rate of A and B to be 0.2ml / min, and start the reaction at a reaction temperature of 40°C in the reactor. After 10 minutes of stable reaction, collect the reaction solution, take the upper layer, and leave it in the refrigerator to precipitate unreacted octanoic acid, filtered, and the filtrate was rotary evaporated, vacuum-dried, and finally white N ε - Octanoyllysine solids, 91% conversion.
Embodiment 2
[0027] Fill 0.495 g of immobilized enzyme Novozyme 435 with a particle size of 0.3 to 0.9 mm into an immobilized enzyme microreactor with an inner diameter of 3.8 mm and a length of 150 mm (retention volume is 1.2 mL), connect the device, and use n-hexane solvent Flush the pipes. The concentration is 80mmol·L -1 The lysine is dissolved in water to obtain the mixed solution A, which is transferred to the device 3, and the concentration is 20mmol·L -1 of capric acid solution Dissolve in n-hexane to obtain mixed solution B and transfer it to device 4, adjust the flow rate of A and B to be 0.2ml / min, start the reaction at the reaction temperature in the reactor at 40°C, collect the reaction solution after the reaction is stable for 10 minutes, and take the upper layer , standing in the refrigerator, unreacted capric acid was separated out, filtered, the filtrate was rotary evaporated, vacuum dried, and finally white N ε - Decanoyl lysine solid, 92% conversion.
Embodiment 3
[0029] Fill 0.495 g of immobilized enzyme Novozyme 435 with a particle size of 0.3 to 0.9 mm into an immobilized enzyme microreactor with an inner diameter of 3.8 mm and a length of 150 mm (retention volume is 1.2 mL), connect the device, and use n-hexane solvent Flush the pipes. The concentration is 80mmol·L -1 The lysine is dissolved in water to obtain the mixed solution A, which is transferred to the device 3, and the concentration is 20mmol·L -1 The lauric acid was dissolved in n-hexane to obtain the mixed solution B, which was transferred to device 4. The flow rate of A and B was adjusted to 0.2ml / min, and the reaction temperature in the reactor was 40°C to start the reaction. After the reaction was stable for 10 minutes, the reaction solution was collected. Take the supernatant, leave it standing in the refrigerator, separate out unreacted lauric acid, filter, spin the filtrate, and dry it in vacuum to get white N ε - Lauroyl lysine solid, 90% conversion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com