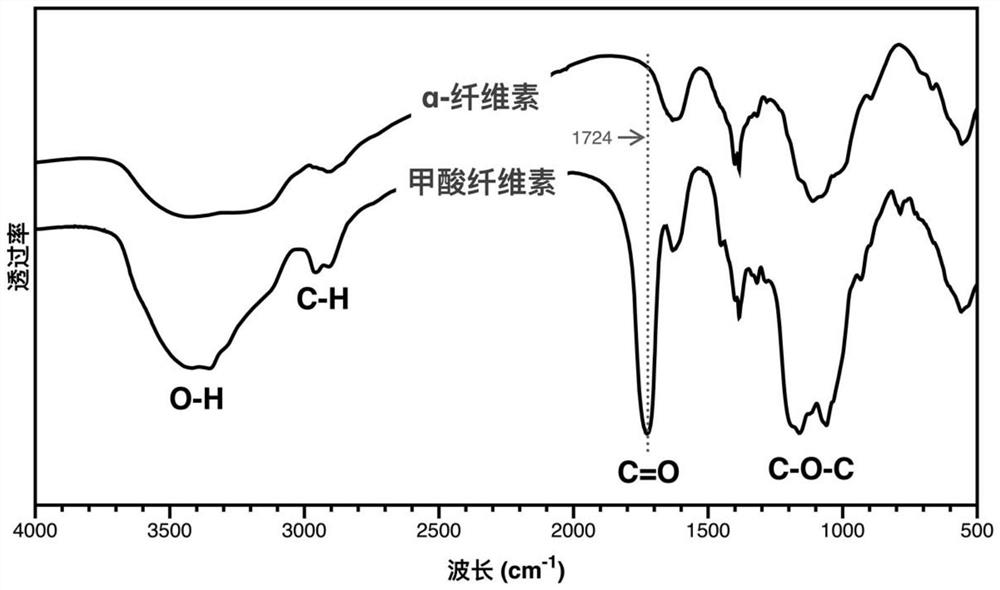
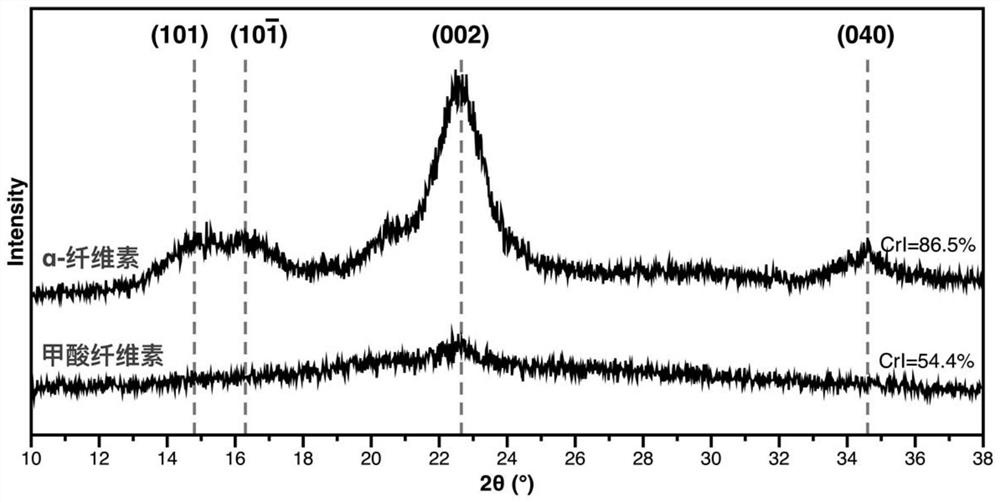
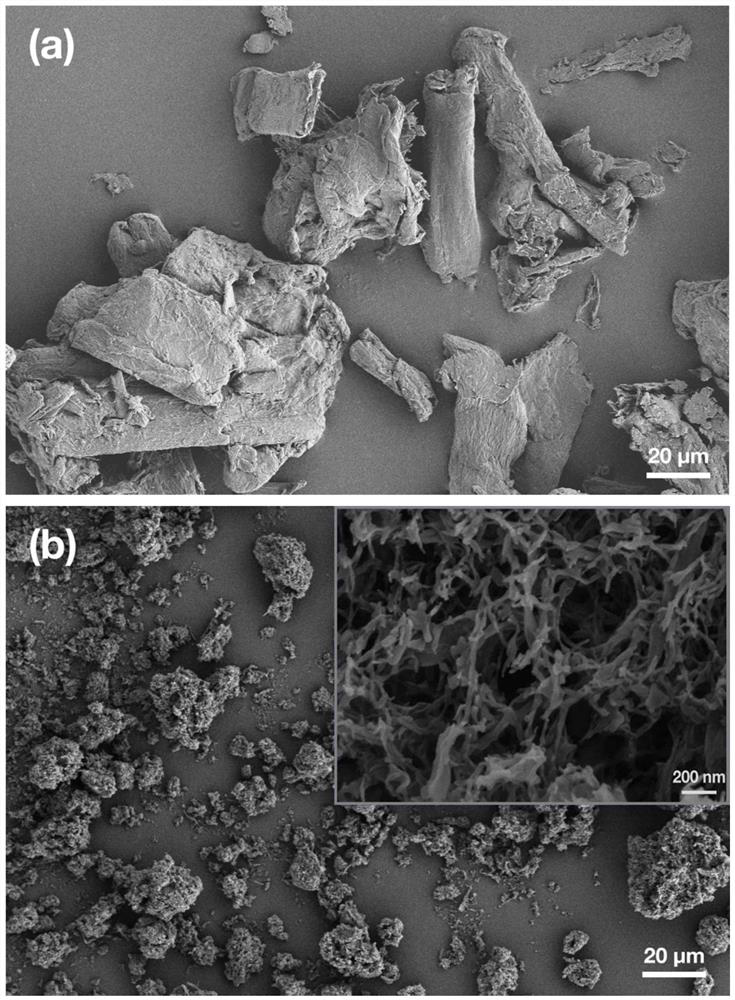
Method for preparing 5-hydroxymethylfurfural by catalyzing cellulose formate with dimethyl sulfoxide-water cosolvent system
A technology of dimethyl sulfoxide and hydroxymethyl furfural, applied in the direction of organic chemistry, etc., can solve the problems of limited dissolving and dispersing ability, unfavorable results, etc., and achieve the effect of excellent directional selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1, formic acid treatment of α-cellulose
[0029] Add 300mL 99% formic acid solution into the round bottom flask and place it in an oil bath to raise the temperature to 120°C, and carry out magnetic stirring (800r / min); weigh 10g of α-cellulose and slowly add it to the formic acid solution, the reaction time is 3h Afterwards, the suspension was centrifuged at 4000r / min for 10min to remove cellulose residues; the reaction filtrate was transferred to a single-necked flask and rotatively evaporated at 45r / min in a water bath at 50°C, and the solution was concentrated to about 10mL; the concentrated The reaction solution was slowly poured into 150mL ethanol solution, the white fine precipitate was precipitated rapidly by magnetic stirring, and it was centrifuged at 10000r / min for 10min, and the white precipitate was obtained by filtration; the white precipitate was then added to ethanol, magnetically stirred, centrifuged (4°C , 10000r / min, 10min), filter, and repeat 3 t...
Embodiment 2
[0033] Step 1, formic acid treatment of α-cellulose
[0034] Add 300mL 99% formic acid solution into the round bottom flask and place it in an oil bath to raise the temperature to 120°C, and carry out magnetic stirring (800r / min); weigh 10g of α-cellulose and slowly add it to the formic acid solution, the reaction time is 3h Afterwards, the suspension was centrifuged at 4000r / min for 10min to remove cellulose residues; the reaction filtrate was transferred to a single-necked flask and rotatively evaporated at 45r / min in a water bath at 50°C, and the solution was concentrated to about 10mL; the concentrated The reaction solution was slowly poured into 150mL ethanol solution, the white fine precipitate was precipitated rapidly by magnetic stirring, and it was centrifuged at 10000r / min for 10min, and the white precipitate was obtained by filtration; the white precipitate was then added to ethanol, magnetically stirred, centrifuged (4°C , 10000r / min, 10min), filter, and repeat 3 t...
Embodiment 3
[0038] Catalytic Preparation of 5-Hydroxymethylfurfural from α-Cellulose
[0039]The catalytic reaction was carried out in a microwave reactor (CEM Discover System) with α-cellulose as substrate. Add 1.25mmol of the substrate into a 10mL quartz tube, add 0.5mL of hydrochloric acid (0.5M), 0.5mL of aluminum chloride (1.0M) as a combined catalyst, and then add 4mL of dimethyl sulfoxide. The final concentrations of the hydrochloric acid and aluminum chloride combined catalysts were 50 mM and 100 mM, respectively. Seal the microwave reaction tube, heat the target reaction temperature to 160°C, and hold the temperature for 20 minutes. After the reaction is finished, cool to room temperature, and measure the content of 5-hydroxymethylfurfural in the filtrate by high performance liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com