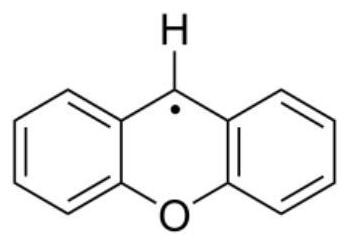
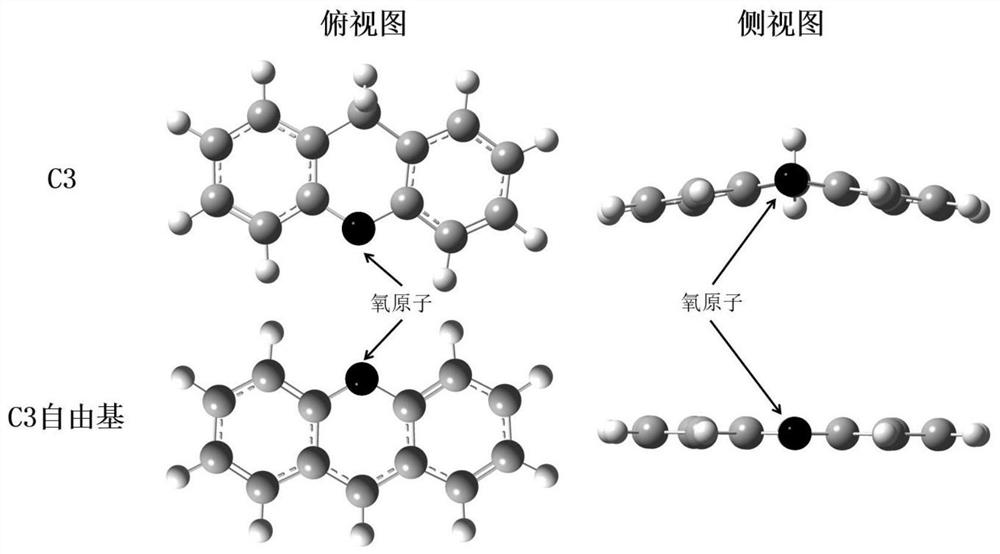
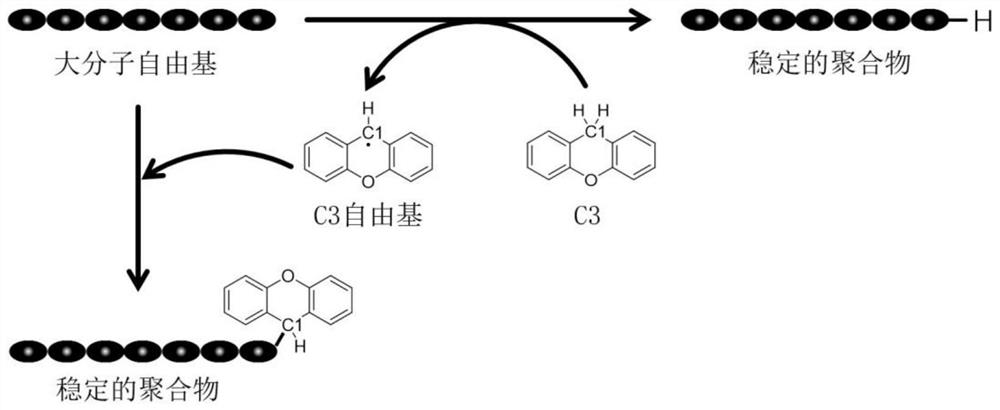
Application of Compounds with Parallel Structures as Controlling Agents for Radical Polymerization
A polymerization reaction and free radical technology, which is applied in the application field of ring-joined compound as a free radical polymerization control agent, can solve the problems of reduced regulation ability of free radical polymerization, easy occurrence of redox reaction, change of final reaction product, etc. Achieve the effect of structure and performance optimization, not easy to deteriorate, high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Add 0.4wt.% of control agent C5 to acrylate monomer ethylene glycol dimethacrylate, and use 1wt% of azobisisobutyronitrile as thermal initiator to initiate thermal polymerization at 60°C, The maximum polymerization reaction rate was close to zero, the gelation time was 4200s, and the degree of functional group reaction was 60% during gelation.
Embodiment 2
[0031] To vinyl monomer N-vinylpyrrolidone and acrylate monomer ethoxylated trimethylolpropane triacrylate (mass ratio 1:3), add 0.6wt.% of control agent C2 to 1wt% The azobisisobutyronitrile is a thermal initiator, which initiates thermal polymerization at 60 °C, the maximum polymerization rate is close to zero, the gelation time is 3000s, and the degree of functional group reaction during gelation is 40%.
Embodiment 3
[0033] Add 1 wt.% of control agent C6 to acrylamide monomer N,N'-diacrylethylenediamine, oxidize with 1 wt.% of phenylbis(2,4,6-trimethylbenzoyl) Phosphine is a photoinitiator, which initiates photopolymerization under ultraviolet light irradiation, and the maximum polymerization rate is 0.025s -1 , the gelation time is 500s, and the degree of functional group reaction is 35% during gelation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com