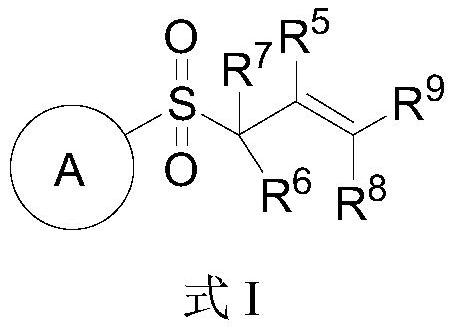
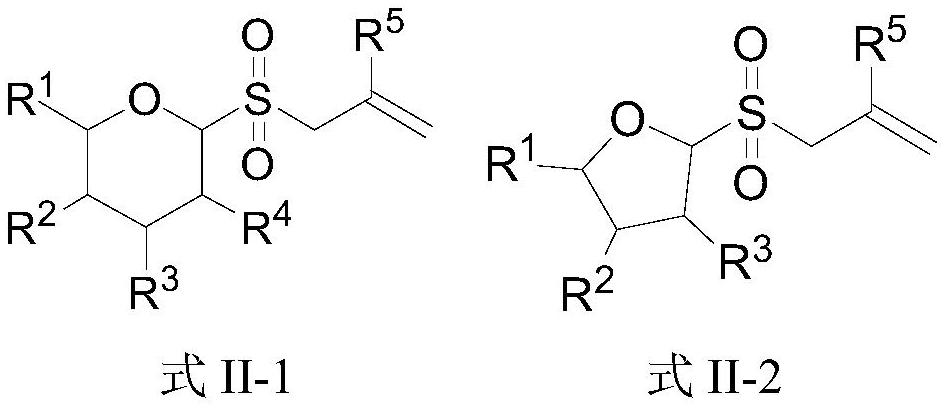
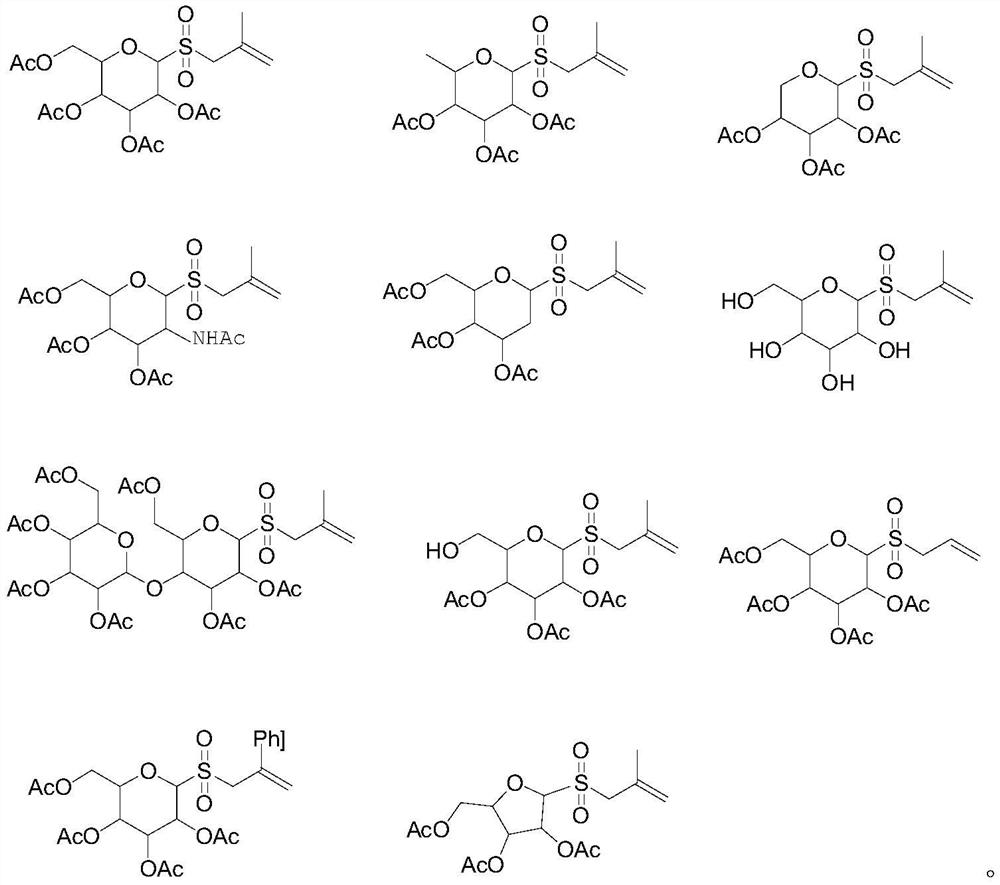
Novel glycosyl donor and thioglycoside compound, and preparation methods of thioglycoside compound
A compound and glycosyl technology, applied in the field of glucosinolate compounds and their preparation, new glycosyl donors, can solve problems such as limited application, difficult peptide and protein modification, complex preparation methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The synthesis of embodiment 1, compound 3, compound 3-x
[0074] 1. According to the following synthetic route, compound 3 is obtained:
[0075]
[0076] Step a: at room temperature, in the dichloromethane solution (wherein the concentration of 3-a is 0.5mol / L) of sugar 3-a (1eqiuv) of all hydroxyl groups, add acetic anhydride (addition amount is the number of hydroxyl groups on 3-a 1.2eqiuv), triethylamine (3eqiuv) and DMAP (0.2eqiuv), stirred overnight at room temperature. After the reaction, the reaction system was washed with saturated potassium carbonate solution, extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered with suction, concentrated, and separated by column chromatography (300 mesh-400 mesh silica gel) to obtain the corresponding all-acetyl-protected product 3-b.
[0077] Step b: Dissolve the fully acetyl-protected product 3-b in acetonitrile (the concentration of 3-b is 0.5mol / L) at room temperature, add thiourea (1.5eqiuv) ...
Embodiment 2
[0085] Embodiment 2, the synthesis of compound 1
[0086]
[0087] 1-Allylsulfone-2,3,4,6-tetraacetyl-α-D-glucopyranose (Yield=75%)
[0088] 1H NMR (400MHz, Chloroform-d) δ5.99–5.79(m,1H),5.58–5.47(m,3H),5.31(t,J=9.3Hz,1H),5.10(t,J=9.8Hz, 1H), 4.56(d, J=9.9Hz, 1H), 4.34–4.16(m, 2H), 3.98(dd, J=13.9, 8.4Hz, 1H), 3.83–3.73(m, 2H), 2.10(s ,3H),2.05(s,3H),2.05(s,3H),2.03(s,3H).
Embodiment 3
[0089] The synthesis of embodiment 3, compound 2
[0090]
[0091] 1-(2-Phenyl-2-propenyl)-sulfone-2,3,4,6-tetraacetyl-α-D-glucopyranose (Yield=71%)
[0092] 1 H NMR (400MHz, Chloroform-d) δ7.50–7.46(m,2H),7.43–7.36(m,3H),5.77(s,1H),5.60(s,1H),5.50(t,J=9.6 Hz, 1H), 5.16(t, J=9.3Hz, 1H), 5.03(t, J=9.8Hz, 1H), 4.51(d, J=14.2Hz, 1H), 4.17(d, J=9.9Hz, 1H), 4.10–4.03(m, 3H), 3.29(dt, J=10.1, 3.5Hz, 1H), 2.10(s, 3H), 2.02(s, 3H), 2.01(s, 3H), 2.00(s ,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com